BCAS1-positive oligodendrocytes enable efficient cortical remyelination in multiple sclerosis
- PMID: 39319704
- PMCID: PMC11884765
- DOI: 10.1093/brain/awae293
BCAS1-positive oligodendrocytes enable efficient cortical remyelination in multiple sclerosis
Abstract
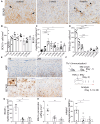
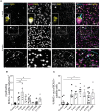
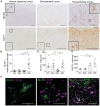
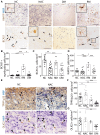
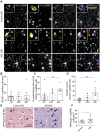
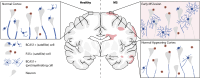
Remyelination is a crucial regenerative process in demyelinating diseases, limiting persisting damage to the CNS. It restores saltatory nerve conduction and ensures trophic support of axons. In patients with multiple sclerosis, remyelination has been observed in both white and grey matter and found to be more efficient in the cortex. Brain-enriched myelin-associated protein 1 (BCAS1) identifies oligodendrocyte lineage cells in the stage of active myelin formation in development and regeneration. Other than in the white matter, BCAS1+ oligodendrocytes are maintained at high densities in the cortex throughout life. Here, we investigated cortical lesions in human biopsy and autopsy tissue from patients with multiple sclerosis in direct comparison to demyelinating mouse models and demonstrate that following a demyelinating insult BCAS1+ oligodendrocytes in remyelinating cortical lesions shift from a quiescent to an activated, internode-forming morphology co-expressing myelin-associated glycoprotein (MAG), necessary for axonal contact formation. Of note, activated BCAS1+ oligodendrocytes are found at early time points of experimental demyelination amidst ongoing inflammation. In human tissue, activated BCAS1+ oligodendrocytes correlate with the density of myeloid cells, further supporting their involvement in an immediate regenerative response. Furthermore, studying the microscopically normal appearing non demyelinated cortex in patients with chronic multiple sclerosis, we find a shift from quiescent BCAS1+ oligodendrocytes to mature, myelin-maintaining oligodendrocytes, suggesting oligodendrocyte differentiation and limited replenishment of BCAS1+ oligodendrocytes in long-standing disease. We also demonstrate that part of perineuronal satellite oligodendrocytes are BCAS1+ and contribute to remyelination in human and experimental cortical demyelination. In summary, our results provide evidence from human tissue and experimental models that BCAS1+ cells in the adult cortex represent a population of pre-differentiated oligodendrocytes that rapidly react after a demyelinating insult thus enabling immediate myelin regeneration. In addition, our data suggest that limited replenishment of BCAS1+ oligodendrocytes may contribute to the remyelination failure observed in the cortex in chronic multiple sclerosis.
Keywords: cortical remyelination; demyelination; multiple sclerosis; oligodendrocytes; satellite cells.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Comment in
-
BCAS1+ oligodendrocytes aid remyelination in MS.Nat Rev Neurol. 2024 Nov;20(11):643. doi: 10.1038/s41582-024-01031-0. Nat Rev Neurol. 2024. PMID: 39402247 No abstract available.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

