Methadone Initiation in the Emergency Department for Opioid Use Disorder
- PMID: 39319796
- PMCID: PMC11418868
- DOI: 10.5811/westjem.18530
Methadone Initiation in the Emergency Department for Opioid Use Disorder
Abstract
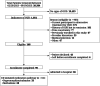
Introduction: Overdose deaths from high-potency synthetic opioids, including fentanyl and its analogs, continue to rise along with emergency department (ED) visits for complications of opioid use disorder (OUD). Fentanyl accumulates in adipose tissue; although rare, this increases the risk of precipitated withdrawal in patients upon buprenorphine initiation. Many EDs have implemented medication for opioid use disorder (MOUD) programs using buprenorphine. However, few offer methadone, a proven therapy without the risk of precipitated withdrawal associated with buprenorphine initiation. We describe the addition of an ED-initiated methadone treatment pathway and compared its 72-hour follow-up outpatient treatment engagement rates to our existing ED-initiated buprenorphine MOUD program.
Methods: We expanded our ED MOUD program with a methadone treatment pathway. From February 20-September 19, 2023, we screened 20,504 ED arrivals; 5.1% had signs of OUD. We enrolled 61 patients: 28 in the methadone; and 33 in the buprenorphine pathways. For patients who screened positive for opioid use, shared decision-making was employed to determine whether buprenorphine or methadone therapy was more appropriate. Patients in the methadone pathway received their first dose of up to 30 milligrams (mg) of methadone in the ED. Two additional methadone doses of up to 40 mg were dispensed at the time of the ED visit and held in the department, allowing patients to return each day for observed dosing until intake at an opioid treatment program (OTP). We compared 72-hour rates of outpatient follow-up treatment engagement at the OTP (for those on methadone) or at the addiction treatment center (ATC) (for those on buprenorphine) for the two treatment pathways.
Results: Of the 28 patients enrolled in the methadone pathway, 12 (43%) successfully engaged in follow-up treatment at the OTP. Of the 33 patients enrolled in the buprenorphine pathway, 15 (45%) successfully engaged in follow-up treatment at the ATC (relative risk 1.06; 95% confidence interval 0.60-1.87).
Conclusion: Methadone initiation in the ED to treat patients with OUD resulted in similar 72-hour follow-up outpatient treatment engagement rates compared to ED-buprenorphine initiation, providing another viable option for MOUD.
Conflict of interest statement
Figures


Similar articles
-
METHADONE INITIATION IN THE EMERGENCY DEPARTMENT FOR OPIOID USE DISORDER: A CASE SERIES.J Emerg Med. 2023 Mar;64(3):391-396. doi: 10.1016/j.jemermed.2023.01.012. J Emerg Med. 2023. PMID: 37019500
-
Opioid Overdose After Medication for Opioid Use Disorder Initiation Following Hospitalization or ED Visit.JAMA Netw Open. 2024 Jul 1;7(7):e2423954. doi: 10.1001/jamanetworkopen.2024.23954. JAMA Netw Open. 2024. PMID: 39037812 Free PMC article.
-
Evaluation of medications used for opioid use disorder in emergency departments: A cross-sectional analysis of the 2020 National Hospital Ambulatory Medical Care Survey.Am J Emerg Med. 2024 Aug;82:52-56. doi: 10.1016/j.ajem.2024.05.015. Epub 2024 May 20. Am J Emerg Med. 2024. PMID: 38795424
-
Management of opioid withdrawal and initiation of medications for opioid use disorder in the hospital setting.Hosp Pract (1995). 2022 Oct;50(4):251-258. doi: 10.1080/21548331.2022.2102776. Epub 2022 Jul 22. Hosp Pract (1995). 2022. PMID: 35837678 Review.
-
Managing emergency department patients with opioid use disorder.Emerg Med Pract. 2024 Jun 1;26(6):1-24. Print 2024 Jun. Emerg Med Pract. 2024. PMID: 38768011 Review.
Cited by
-
Rapid Titration of Methadone for Opioid Use Disorder in the Emergency Department: A Case Report.Clin Pract Cases Emerg Med. 2025 May;9(2):188-192. doi: 10.5811/cpcem.39968. Clin Pract Cases Emerg Med. 2025. PMID: 40402058 Free PMC article.
References
-
- Spencer MR, Miniño AM, Warner M. Drug overdose deaths in the United States, 2001–2021. 2022. Available at: https://www.cdc.gov/nchs/products/databriefs/db457.htm. Accessed March 23, 2023. - PubMed
-
- National Institute on Drug Abuse . Drug overdose death rates. 2023. Available at: https://nida.nih.gov/research-topics/trends-statistics/overdose-death-rates. Accessed March 23, 2023.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
