Fondaparinux versus Enoxaparin in the Treatment of Obese Patients with Acute Coronary Syndrome
- PMID: 39319877
- PMCID: PMC11495804
- DOI: 10.36660/abc.20230793
Fondaparinux versus Enoxaparin in the Treatment of Obese Patients with Acute Coronary Syndrome
Abstract
Background: Fondaparinux is an effective and safe anticoagulant in the treatment of acute coronary syndromes (ACS). However, due to the low representation of obese individuals in clinical trials, the effects of applying the results of this drug to this population remain uncertain.
Objectives: To compare Fondaparinux to Enoxaparin in the treatment of obese patients with ACS.
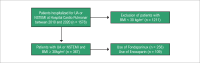
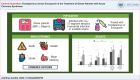
Methods: This is a retrospective cohort study, including obese individuals (BMI ≥ 30 Kg/m2) admitted with non-ST-segment elevation myocardial infarction (NSTEMI) or unstable angina (UA) and treated with Fondaparinux or Enoxaparin between 2010 and 2020. The Fondaparinux and Enoxaparin groups were compared for their clinical and laboratory characteristics using chi-square and Mann-Whitney tests, as appropriate. The incidence of primary outcomes (death, reinfarction, stroke, major bleeding) was compared between groups. P-value < 0.05 was considered significant for all analyses.
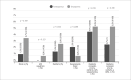
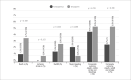
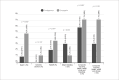
Results: A total of 367 obese patients with NSTEMI or UA were included, of whom 258 used Fondaparinux and 109 used Enoxaparin. Mean age was 64 ± 12 years, and 52.9% were male. The prevalence of diabetes, hypertension, dyslipidemia, prior coronary artery disease, prior stroke, and implementation of invasive strategy was similar between groups. The incidence of the primary outcome was 4.7% in the Fondaparinux group and 5.5% in the Enoxaparin group (p = 0.729). There was no difference between groups when analyzing the components of the primary outcome separately.
Conclusion: In a sample of obese patients with NSTEMI or UA, there was no difference in the occurrence of the composite outcome (death, stroke, reinfarction, major bleeding) between patients who used Fondaparinux or Enoxaparin.
Fundamento: O fondaparinux é um anticoagulante eficaz e seguro usado no tratamento de síndromes coronarianas agudas (SCAs). No entanto, devido à baixa representatividade de indivíduos obesos em ensaios clínicos, os efeitos de se aplicar os resultados desse medicamento nesta população continuam incertos.
Objetivos: Comparar o fondaparinux à enoxaparina no tratamento de obesos com SCA.
Métodos: Este é um estudo do tipo coorte retrospectivo, incluindo indivíduos obesos (IMC ≥ 30 Kg/m2) internados com Infarto do Miocárdio sem Elevação do Segmento ST (IAMSSST) ou Angina Instável (AI) e tratados com fondaparinux ou enoxaparina entre 2010 e 2020. Os grupos que receberam fondaparinux e enoxaparina foram comparados quanto suas características clínicas e laboratoriais usando o teste do qui-quadrado e o teste de Mann-Whitney, conforme apropriado. A incidência dos desfechos primários (morte, reinfarto, acidente vascular cerebral, sangramento maior) foi comparada entre os grupos. Um p<0,05 foi considerado estatisticamente significativo em todas as análises.
Resultados: Um total de 367 pacientes obesos com IAMSSST ou AI foi incluído, dos quais 258 usaram fondaparinux e 109 usaram enoxaparina. A idade média foi 64 ± 12 anos, 52,9% eram do sexo masculino. A prevalência e diabetes, hipertensão, dislipidemia, doença arterial coronariana prévia, acidente vascular cerebral prévio, e implementação de estratégia invasiva foi similar entre os grupos. A incidência do desfecho primário foi 4,7% no grupo fondaparinux e 5,5% no grupo enoxaparina (p = 0,729). Não houve diferença entre os grupos quando os componentes do desfecho primário foram analisados separadamente.
Conclusão: Em uma amostra de pacientes obesos com IAMSSST ou AI, não houve diferença na ocorrência do desfecho composto (morte, acidente vascular cerebral, reinfarto, sangramento maior) entre os pacientes que utilizaram fondaparinux ou enoxaparina.
Conflict of interest statement
Figures



References
-
- World Health Organization . The Top 10 Causes of Death, 2000-2019 [Internet] Geneva: World Health Organization; 2020. [[cited 2024 Jan 17]]. Available from: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

