Prevalence, prognosis, and health care resource utilization in carriers of pathogenic germline variants in BRCA1/2 with incident early-stage breast cancer: a Finnish population-based study
- PMID: 39319938
- PMCID: PMC11445600
- DOI: 10.2340/1651-226X.2024.40829
Prevalence, prognosis, and health care resource utilization in carriers of pathogenic germline variants in BRCA1/2 with incident early-stage breast cancer: a Finnish population-based study
Abstract
Background and purpose: Data on real-world prevalence and outcomes in patients diagnosed with pathogenic germline variants in BRCA1 or BRCA2 (gBRCAm) breast cancer is sparse.
Material and methods: An observational cohort study including all patients diagnosed with incident early-stage breast cancer and recorded in Helsinki University Hospital data lake 2012-2022, accounting for one-third of the Finnish breast cancer patient population.
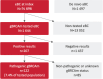
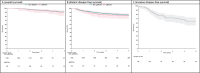
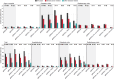
Results: Among 14,696 incident early-stage breast cancer patients, 11.2% (n = 1,644) were tested for gBRCAm. Of the tested population, 7.4% (n = 122) carried gBRCAm. Of the 122 gBRCAm patients, 95.1% (n = 116) were women, with a median age at diagnosis of 46.4 years. Among the same patient group, HER2 status was available for 87.7% (n = 107) of the patients. Among these, 49.5% (n = 53) had hormone receptor-positive (HR+), HER2-negative breast cancer, 13.1% were (n = 14) HER2-positive, and 37.3% (n = 40) of patients had triple-negative breast cancer. The tested patients were significantly younger compared with non-tested patients. No significant differences in overall survival or healthcare resource utilization between the tested patients with gBRCAm and gBRCA wild-type (gBRCAwt) were observed.
Interpretation: This comprehensive observational study supports previous findings of gBRCAm prevalence in the Western early-stage breast cancer population. While no differences in survival were observed between patients with gBRCAm and gBRCAwt, it is important to consider the potential influence of selection bias, particularly due to the younger gBRCAm testing target population and the overall low frequency of testing. Therefore, a substantial proportion of the patients carrying gBRCAm likely remained undiagnosed, and wider screening criteria are warranted.
Conflict of interest statement
PK has received a grant from AstraZeneca for this project. TÅ and RE are employees of AstraZeneca.
Figures

Similar articles
-
Prevalence of germline BRCA mutations in HER2-negative metastatic breast cancer: global results from the real-world, observational BREAKOUT study.Breast Cancer Res. 2020 Oct 27;22(1):114. doi: 10.1186/s13058-020-01349-9. Breast Cancer Res. 2020. PMID: 33109210 Free PMC article. Clinical Trial.
-
Clinical Outcomes, Treatment Patterns, and Health Resource Utilization Among Metastatic Breast Cancer Patients with Germline BRCA1/2 Mutation: A Real-World Retrospective Study.Adv Ther. 2019 Mar;36(3):708-720. doi: 10.1007/s12325-018-0867-x. Epub 2019 Jan 17. Adv Ther. 2019. PMID: 30656571
-
Efficacy of front-line treatment for hormone receptor-positive HER2-negative metastatic breast cancer with germline BRCA1/2 mutation.Br J Cancer. 2023 Jun;128(11):2072-2080. doi: 10.1038/s41416-023-02248-4. Epub 2023 Apr 3. Br J Cancer. 2023. PMID: 37012318 Free PMC article.
-
A systematic review and meta-analysis of germline BRCA mutations in pancreatic cancer patients identifies global and racial disparities in access to genetic testing.ESMO Open. 2023 Apr;8(2):100881. doi: 10.1016/j.esmoop.2023.100881. Epub 2023 Feb 21. ESMO Open. 2023. PMID: 36822114 Free PMC article.
-
BRCA-mutated breast cancer: the unmet need, challenges and therapeutic benefits of genetic testing.Br J Cancer. 2024 Nov;131(9):1400-1414. doi: 10.1038/s41416-024-02827-z. Epub 2024 Aug 30. Br J Cancer. 2024. PMID: 39215191 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

