Reversibility of cognitive worsening observed with BACE inhibitor umibecestat in the Alzheimer's Prevention Initiative (API) Generation Studies
- PMID: 39320017
- PMCID: PMC11567862
- DOI: 10.1002/alz.14237
Reversibility of cognitive worsening observed with BACE inhibitor umibecestat in the Alzheimer's Prevention Initiative (API) Generation Studies
Abstract
Introduction: The Alzheimer's Prevention Initiative (API) Generation Studies evaluated the BACE inhibitor umibecestat for Alzheimer's disease (AD) prevention. The studies were terminated early, and the reversibility of umibecestat's side effects was assessed.
Methods: Cognitively unimpaired 60- to 75-year-old apolipoprotein E (APOE) ε4 homozygotes and heterozygotes (the latter with elevated brain amyloid deposition) (n = 1556) received umibecestat (50 or 15 mg daily) or placebo for 7 months on average and were followed for a median (interquartile range) of 4 (3 to 6) months after washout.
Results: Compared to placebo, umibecestat-treated participants had small, non-progressive, but statistically significant decline in performance on certain cognitive batteries including Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) and API Preclinical Composite Cognitive test, but not Clinical Dementia Rating-Sum of Boxes. RBANS differences were no longer significant at the end of follow-up.
Discussion: In people at genetic risk for AD, high-dose beta-site amyloid precursor protein cleaving enzyme (BACE) inhibition was associated with early mild cognitive worsening, which reversed shortly after washout, suggesting a symptomatic side effect not associated with neurodegeneration. Fully anonymized data, images, and samples are available upon request for further research on BACE inhibition.
Highlights: This is the first trial with blinded assessment of reversibility of BACE inhibitor side effects. Umibecestat was tested in cognitively unimpaired persons at genetic risk for AD. Umibecestat led to early mild cognitive decline that reversed shortly after washout. This suggests a potentially manageable effect not associated with neurodegeneration. Further research may determine the future of BACE inhibition in AD prevention.
Keywords: APOE; Alzheimer's disease (AD) prevention; BACE inhibitors; amyloid beta lowering; biomarkers; imaging.
© 2024 Novartis Pharma AG. Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
Stephen Salloway has received grants from Biogen, Eisai, Genentech, Roche, Novartis, and Eli Lilly; personal fees from Biogen, Eisai, Eli Lilly, Novartis, Genentech, NovoNordisk, Prothena, AbbVie, Kisbee, Acumen, CognitionRX, and Roche; non‐financial support from Biogen, Lilly, Acumen, Abbvie, and Roche; and was a site investigator for the Generation studies and co‐chair of the investigator steering committee for the ENGAGE study. Jeffrey M. Burns has received research support to conduct clinical trials (paid to his institution) from Eli Lilly, Amylyx Pharmaceuticals, Biogen, AbbVie, AstraZeneca, and Roche and has served as a consultant for Renew Research, Amylyx Pharmaceuticals, Eisai, Eli Lilly, Labcorp, New Amsterdam Pharma, and Renew Biotechnologies. Jón G. Snaeda has received consulting fees from Novartis. Vissia Viglietta was a full‐time employee of Amgen at the time of the study and is currently an employee of Nido Biosciences, which was not involved in this study. Pierre N. Tariot, Eric M. Reiman, and Jessica B. Langbaum are full‐time employees of Banner Health. Banner Health received financial support from Novartis Pharma AG and Amgen for the conduct of the API Generation Program, from Eli Lilly for the conduct of another Alzheimer's prevention trial, and from Genentech/Roche for another Alzheimer's prevention trial. Pierre N. Tariot reports receiving grants from the National Institute on Aging (NIA) (UF1AG046150, RF1 AG041705, R01AG055444, and 1R01AG058468) and received consulting fees from AbbVie, AC Immune, Acadia, Axsome, Biogen, BioXcel, Cortexyme, Eisai, Genentech, Lundbeck, Otsuka & Astex, Merck & Co., Novo Nordisk, Syneos, and T3D Therapeutics. Eric M. Reiman reports receiving grants from the NIA (1UF1AG046150, RF1AG041705, R01AG05544, 1R01AG058468, P30AG19610, and P30AG072980). He is a compensated scientific advisor for Alzheon, Aural Analytics, Denali, Retromer Therapeutics, and Vaxxinity and an uncompensated advisor to Biogen and Eli Lilly. He is a co‐founder, advisor, and shareholder in ALZPath. Jessica B. Langbaum reports receiving grants from NIA (1UF1AG046150, RF1AG041705, R01AG05544, 1R01AG058468, and P30AG072980) and received consulting fees from Alector, Biogen, Denovo Biopharma, and Provoc. Marie‐Emmanuelle Riviere, Beth Borowsky, Fonda Liu, Marie‐Laure Rouzade‐Dominguez, Pilar Cazorla, Marie‐Catherine Mousseau, Michal Arkuszewski, Javier Ricart, Yihan Sui, Angelika Caputo, and Ana Graf are employees of and shareholders in Novartis. This research was sponsored by Novartis Pharma AG, Basel, Switzerland. Cristina Lopez Lopez was a full‐time employee of Novartis Pharma AG at the time of the study and is currently an employee of Roche, which was not involved in this study. Author disclosures are available in the supporting information.
Figures


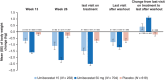

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

