Sleep state-dependent development of resting-state functional connectivity during the preterm period
- PMID: 39320057
- PMCID: PMC11632190
- DOI: 10.1093/sleep/zsae225
Sleep state-dependent development of resting-state functional connectivity during the preterm period
Abstract
Study objectives: The brains of preterm infants exhibit altered functional connectivity (FC) networks, but the potential variation in sleep states and the impact of breathing patterns on FC networks are unclear. This study explores the evolution of resting-state FC from preterm to term, focusing on breathing patterns and distinguishing between active sleep (AS) and quiet sleep (QS).
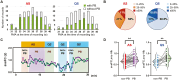
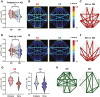
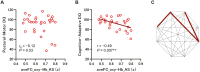
Methods: We recruited 63 preterm infants and 44 healthy-term infants and performed simultaneous electroencephalography and functional near-infrared spectroscopy. FC was calculated using oxy- and deoxyhemoglobin signals across eight channels. First, FC was compared between periodic breathing (PB) and non-PB segments. Then sleep state-dependent FC development was explored. FC was compared between AS and QS segments and between preterm infants at term and term-born infants in each sleep state. Finally, associations between FC at term, clinical characteristics, and neurodevelopmental outcomes in late infancy were assessed in preterm infants.
Results: In total, 148 records from preterm infants and 44 from term-born infants were analyzed. PB inflated FC values. After excluding PB segments, FC was found to be elevated during AS compared to QS, particularly in connections involving occipital regions. Preterm infants had significantly higher FC in both sleep states compared to term-born infants. Furthermore, stronger FC in specific connections during AS at term was associated with unfavorable neurodevelopment in preterm infants.
Conclusions: Sleep states play a critical role in FC development and preterm infants show observable changes in FC.
Keywords: electroencephalography; functional connectivity; near-infrared spectroscopy; outcome; periodic breathing; preterm infant; sleep state.
© The Author(s) 2024. Published by Oxford University Press on behalf of Sleep Research Society.
Figures



References
-
- Luhmann HJ, Kanold PO, Molnár Z, Vanhatalo S.. Early brain activity: translations between bedside and laboratory. Prog Neurobiol. 2022;213:102268. doi: https://doi.org/10.1016/j.pneurobio.2022.102268 - DOI - PMC - PubMed
-
- Molnar Z, Luhmann HJ, Kanold PO.. Transient cortical circuits match spontaneous and sensory-driven activity during development. Science. 2020;370(6514):eabb2153. doi: https://doi.org/10.1126/science.abb2153 - DOI - PMC - PubMed
-
- Inder TE, Volpe JJ, Anderson PJ.. Defining the neurologic consequences of preterm birth. N Engl J Med. 2023;389(5):441–453. doi: https://doi.org/10.1056/NEJMra2303347 - DOI - PubMed
-
- Pierrat V, Marchand-Martin L, Arnaud C, et al.; EPIPAGE-2 writing group. Neurodevelopmental outcome at 2 years for preterm children born at 22 to 34 weeks’ gestation in France in 2011: EPIPAGE-2 cohort study. BMJ. 2017;358:j3448. doi: https://doi.org/10.1136/bmj.j3448 - DOI - PMC - PubMed
-
- Cheong JLY, Olsen JE, Lee KJ, et al.; Victorian Infant Collaborative Study Group. Temporal trends in neurodevelopmental outcomes to 2 years after extremely preterm birth. JAMA Pediatr. 2021;175(10):1035–1042. doi: https://doi.org/10.1001/jamapediatrics.2021.2052 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

