Virulence of Burkholderia pseudomallei ATS2021 Unintentionally Imported to United States in Aromatherapy Spray
- PMID: 39320153
- PMCID: PMC11431913
- DOI: 10.3201/eid3010.240084
Virulence of Burkholderia pseudomallei ATS2021 Unintentionally Imported to United States in Aromatherapy Spray
Abstract
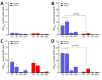
In the United States in 2021, an outbreak of 4 cases of Burkholderia pseudomallei, the etiologic agent of melioidosis and a Tier One Select Agent (potential for deliberate misuse and subsequent harm), resulted in 2 deaths. The causative strain, B. pseudomallei ATS2021, was unintentionally imported into the United States in an aromatherapy spray manufactured in India. We established that ATS2021 represents a virulent strain of B. pseudomallei capable of robust formation of biofilm at physiologic temperatures that may contribute to virulence. By using mouse melioidosis models, we determined median lethal dose estimates and analyzed the bacteriologic and histopathologic characteristics of the organism, particularly the potential neurologic pathogenesis that is probably associated with the bimABm allele identified in B. pseudomallei strain ATS2021. Our data, combined with previous case reports and the identification of endemic B. pseudomallei strains in Mississippi, support the concept that melioidosis is emerging in the United States.
Keywords: ATS2021; Burkholderia pseudomallei; Indian strain; United States; bacteria; biofilm; imported; melioidosis; mice; neurologic.
Figures






References
-
- Chantratita N, Phunpang R, Yarasai A, Dulsuk A, Yimthin T, Onofrey LA, et al. Characteristics and one year outcomes of melioidosis patients in northeastern Thailand: a prospective, multicenter cohort study. Lancet Reg Health Southeast Asia. 2023;9:9. 10.1016/j.lansea.2022.100118 - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

