Temporal Characterization of Prion Shedding in Secreta of White-Tailed Deer in Longitudinal Study of Chronic Wasting Disease, United States
- PMID: 39320164
- PMCID: PMC11431932
- DOI: 10.3201/eid3010.240159
Temporal Characterization of Prion Shedding in Secreta of White-Tailed Deer in Longitudinal Study of Chronic Wasting Disease, United States
Abstract
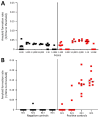
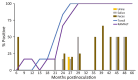
Chronic wasting disease (CWD) affects cervids in North America, Asia, and Scandinavia. CWD is unique in its efficient spread, partially because of contact with infectious prions shed in secreta. To assess temporal profiles of CWD prion shedding, we collected saliva, urine, and feces from white-tailed deer for 66 months after exposure to low oral doses of CWD-positive brain tissue or saliva. We analyzed prion seeding activity by using modified amyloid amplification assays incorporating iron oxide bead extraction, which improved CWD detection and reduced false positives. CWD prions were detected in feces, urine, and saliva as early as 6 months postinfection. More frequent and consistent shedding was observed in deer homozygous for glycine at prion protein gene codon 96 than in deer expressing alternate genotypes. Our findings demonstrate that improved amplification methods can be used to identify early antemortem CWD prion shedding, which might aid in disease surveillance of cervids.
Keywords: CWD; IPQ; RT-QuIC; United States; chronic wasting disease; excreta; feces; prions; prions and related diseases; real-time quaking induced conversion; saliva; secreta; urine; white-tailed deer.
Figures


Similar articles
-
Longitudinal Detection of Prion Shedding in Saliva and Urine by Chronic Wasting Disease-Infected Deer by Real-Time Quaking-Induced Conversion.J Virol. 2015 Sep;89(18):9338-47. doi: 10.1128/JVI.01118-15. Epub 2015 Jul 1. J Virol. 2015. PMID: 26136567 Free PMC article.
-
Rapid antemortem detection of CWD prions in deer saliva.PLoS One. 2013 Sep 11;8(9):e74377. doi: 10.1371/journal.pone.0074377. eCollection 2013. PLoS One. 2013. PMID: 24040235 Free PMC article.
-
Longitudinal detection of prion shedding in nasal secretions of CWD-infected white-tailed deer.J Gen Virol. 2023 Jan;104(1):001825. doi: 10.1099/jgv.0.001825. J Gen Virol. 2023. PMID: 36748533 Free PMC article.
-
Chronic wasting disease of cervids.Curr Top Microbiol Immunol. 2004;284:193-214. doi: 10.1007/978-3-662-08441-0_8. Curr Top Microbiol Immunol. 2004. PMID: 15148993 Review.
-
Chronic wasting disease.Biochim Biophys Acta. 2007 Jun;1772(6):610-8. doi: 10.1016/j.bbadis.2006.10.010. Epub 2006 Oct 18. Biochim Biophys Acta. 2007. PMID: 17223321 Free PMC article. Review.
Cited by
-
Relationship Between Chronic Wasting Disease (CWD) Infection and Pregnancy Probability in Wild Female White-Tailed Deer (Odocoileus virginianus) in Northern Illinois, USA.Pathogens. 2025 Aug 7;14(8):786. doi: 10.3390/pathogens14080786. Pathogens. 2025. PMID: 40872296 Free PMC article.
-
Enhanced detection of chronic wasting disease in muscle tissue harvested from infected white-tailed deer employing combined prion amplification assays.PLoS One. 2024 Oct 23;19(10):e0309918. doi: 10.1371/journal.pone.0309918. eCollection 2024. PLoS One. 2024. PMID: 39441867 Free PMC article.
-
Vertical transmission of chronic wasting disease in free-ranging white-tailed deer populations.Sci Rep. 2025 Aug 5;15(1):28553. doi: 10.1038/s41598-025-12727-8. Sci Rep. 2025. PMID: 40764633 Free PMC article.
-
Cases of Creutzfeldt-Jakob disease in young individuals: open questions regarding aetiology.Front Cell Neurosci. 2025 Apr 14;19:1571662. doi: 10.3389/fncel.2025.1571662. eCollection 2025. Front Cell Neurosci. 2025. PMID: 40297707 Free PMC article. No abstract available.
-
The Evolution of Experimental Rodent Models for Prion Diseases.J Neurochem. 2025 Mar;169(3):e70039. doi: 10.1111/jnc.70039. J Neurochem. 2025. PMID: 40108932 Review.
References
-
- US Geological Survey, National Wildlife Health Center. Expanding distribution of chronic wasting disease. 2022. [cited 2024 Jun 1]. https://www.usgs.gov/centers/nwhc/science/expanding-distribution-chronic...
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

