Initial development of a rapid, portable, stall-side ELISA for the measurement of equine adrenocorticotropic hormone
- PMID: 39320416
- PMCID: PMC11559900
- DOI: 10.1177/10406387241285453
Initial development of a rapid, portable, stall-side ELISA for the measurement of equine adrenocorticotropic hormone
Abstract
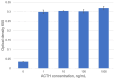
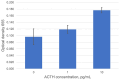
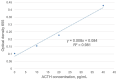
Pituitary pars intermedia dysfunction (PPID) is a neurodegenerative disease of senior horses. Loss of dopaminergic inhibition of the melanotropes of the pars intermedia leads to increased concentrations of pro-opiomelanocortin (POMC)-derived peptides. Diagnosis is challenging due to pre-analytical variables, such as sample storage, handling, and time to analysis. Our objective was to develop an ELISA for ACTH measurement, which could ultimately form the basis for a stall-side equine ACTH test. We selected 2 ACTH-specific monoclonal antibodies, CBL57 and EPR20361-248, based on the recognition of separate epitopes, strong and rapid color change, and minimal background interference, including no cross-reactivity with themselves, each other, and the test reagents. CBL57 was chosen as the detection antibody (or secondary antibody). EPR20361-248, functionalized on superparamagnetic iron oxide beads, was chosen as the capture antibody (or primary antibody) to bind ACTH in plasma. The incorporation of magnetic beads marks the initial stage in establishing a platform that could potentially be utilized in the field, similar to other stall-side tests. The concentrations of antibodies, magnetic beads, and incubation durations were optimized. Our immunoassay detected unglycosylated rat recombinant ACTH. Further studies are ongoing to optimize and validate our assay using equine plasma and serum samples.
Keywords: ACTH; ELISA; equine Cushing disease; horses; pituitary pars intermedia dysfunction.
Conflict of interest statement
Declaration of conflicting interestsThe authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
Similar articles
-
The effect of freeze-thaw cycles on determination of immunoreactive plasma adrenocorticotrophic hormone concentrations in horses.J Vet Intern Med. 2020 May;34(3):1350-1356. doi: 10.1111/jvim.15771. Epub 2020 Apr 7. J Vet Intern Med. 2020. PMID: 32255541 Free PMC article.
-
BEVA primary care clinical guidelines: Diagnosis and management of equine pituitary pars intermedia dysfunction.Equine Vet J. 2024 Mar;56(2):220-242. doi: 10.1111/evj.14009. Epub 2023 Oct 5. Equine Vet J. 2024. PMID: 37795557 Review.
-
Circannual variation in plasma adrenocorticotropic hormone concentrations in the UK in normal horses and ponies, and those with pituitary pars intermedia dysfunction.Equine Vet J. 2012 Jul;44(4):440-3. doi: 10.1111/j.2042-3306.2011.00444.x. Epub 2011 Aug 18. Equine Vet J. 2012. PMID: 21848531
-
Investigation of single and paired measurements of adrenocorticotropic hormone for the diagnosis of pituitary pars intermedia dysfunction in horses.J Vet Intern Med. 2015 Jan;29(1):355-61. doi: 10.1111/jvim.12489. Epub 2014 Oct 13. J Vet Intern Med. 2015. PMID: 25312676 Free PMC article.
-
Diagnosis of equine pituitary pars intermedia dysfunction.Vet J. 2023 Oct-Dec;300-302:106036. doi: 10.1016/j.tvjl.2023.106036. Epub 2023 Oct 6. Vet J. 2023. PMID: 37805159 Review.
References
-
- Equine Endocrinology Group. Recommendations for the diagnosis and management of pituitary pars intermedia dysfunction (PPID). 2023. https://equineendocrinologygroup.org/s/2023-EEG-PPID-Digital-version-wit...
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

