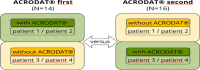
Shared decision-making and detection of comorbidities in an online acromegaly consultation with and without the Acromegaly Disease Activity Tool ACRODAT® using the simulated person approach
- PMID: 39320650
- PMCID: PMC11513722
- DOI: 10.1007/s11102-024-01460-6
Shared decision-making and detection of comorbidities in an online acromegaly consultation with and without the Acromegaly Disease Activity Tool ACRODAT® using the simulated person approach
Abstract
Objective: A patient-centered approach to the management of acromegaly includes disease activity control, shared decision-making and identification of comorbidities. The Acromegaly Disease Activity Tool (ACRODAT®) is intended to assist physicians in providing such holistic management. The present study investigated this claim using the simulated person (SP) approach.
Methods: We studied patient-doctor interaction via online video consultation in a randomized prospective study design with SPs trained to simulate a specific acromegaly profile. We analyzed the proportion of conversation time devoted to health content and the specific acromegaly and comorbidity relevant categories mentioned in the conversation. We collected physicians' feedback on the usefulness of ACRODAT®, SPs subjective perception of the quality of the conversation and compared consultations with and without ACRODAT® using a qualitative approach.
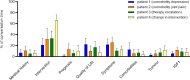
Results: The sample (N = 30) consisted of endocrinologists treating patients with acromegaly in Germany. For SP-physician interactions (N = 60), the proportion of time spent on conversation content (e.g. IGF-I, quality of life) was distributed according to the focus of the patient profile. Comorbidities were less well identified than the need for a change in therapy. Only 18.3% of the SPs were actively asked to participate in the decision-making process. ACRODAT® did not lead to any significant differences in the course of the discussion.
Conclusions: Shared decision-making was underrepresented in this SP-physician interaction in acromegaly management. Physicians adapted the content of the discussion to the SP's needs, but did not adequately address comorbidities. According to the analysis criteria used, ACRODAT® did not contribute to a more holistic patient management in the present study.
Keywords: ACRODAT®; Acromegaly; Comorbidity; Shared decision-making; Simulation person.
© 2024. The Author(s).
Conflict of interest statement
IKA and NU and JH received research support and/or travel grants and/or lecture or advisory board honoraria from Recordati Rare Diseases GbmH Germany, IPSEN Pharma GmbH Germany, Advanz Pharma GmbH, Pfizer Pharma GmbH Germany and Pfizer Co., all of which are manufactures of acromegaly medications. SoS received research support and/or travel grants and/or consultation fees from IPSEN Pharma GmbH Germany, Advanz Pharma GmbH, Pfizer Pharma GmbH Germany and Pfizer Inc. BH received research support and/or travel grants and/or lecture or advisory board honoraria from Recordati Rare Diseases GmbH, Pfizer Pharma GmbH Germany and Alexion Pharma Germany GmbH. LS, ALF, AHF and PD declare to have no conflict of interest in relation to the content of this article.
Figures
Similar articles
-
Acromegaly disease activity according to ACRODAT®, a cross-sectional study in Spain: ACROVAL study.Endocrine. 2022 Feb;75(2):525-536. doi: 10.1007/s12020-021-02900-0. Epub 2021 Oct 19. Endocrine. 2022. PMID: 34668173 Free PMC article.
-
Development of ACRODAT®, a new software medical device to assess disease activity in patients with acromegaly.Pituitary. 2017 Dec;20(6):692-701. doi: 10.1007/s11102-017-0835-5. Pituitary. 2017. PMID: 28887782 Free PMC article.
-
AcroVoice: eliciting the patients' perspective on acromegaly disease activity.Pituitary. 2019 Feb;22(1):62-69. doi: 10.1007/s11102-018-00933-9. Pituitary. 2019. PMID: 30627944 Free PMC article.
-
Evaluating the Impact of Acromegaly on Quality of Life.Endocrinol Metab Clin North Am. 2022 Dec;51(4):709-725. doi: 10.1016/j.ecl.2022.04.004. Epub 2022 Sep 22. Endocrinol Metab Clin North Am. 2022. PMID: 36244688 Review.
-
Effective follow-up consultations: the importance of patient-centered communication and shared decision making.Paediatr Respir Rev. 2013 Dec;14(4):224-8. doi: 10.1016/j.prrv.2013.01.002. Epub 2013 Feb 20. Paediatr Respir Rev. 2013. PMID: 23434177 Review.
Cited by
-
ACRODAT and SAGIT for the assessment of disease activity in acromegaly: a multicenter study of the Veneto region in Italy.Pituitary. 2025 Jun 22;28(4):76. doi: 10.1007/s11102-025-01543-y. Pituitary. 2025. PMID: 40544422 No abstract available.
References
-
- Fleseriu M et al (2022) Acromegaly: pathogenesis, diagnosis, and management. Lancet Diabetes Endocrinol 10(11):804–826. 10.1016/s2213-8587(22)00244-3 - PubMed
-
- Kreitschmann-Andermahr I et al (2017) Predictors of quality of life In 165 patients with acromegaly: Results From a Single-center study. Endocr Pract 23(1):79–88. 10.4158/ep161373.Or - PubMed
-
- Siegel S et al (2021) Illness-related burden, personal resources and need for support in patients with acromegaly: results of a focus group analysis. Growth Horm IGF Res 60–61. 10.1016/j.ghir.2021.101422 - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources