ATXN3: a multifunctional protein involved in the polyglutamine disease spinocerebellar ataxia type 3
- PMID: 39320846
- PMCID: PMC11440613
- DOI: 10.1017/erm.2024.10
ATXN3: a multifunctional protein involved in the polyglutamine disease spinocerebellar ataxia type 3
Abstract
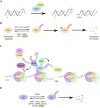
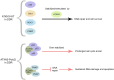
ATXN3 is a ubiquitin hydrolase (or deubiquitinase, DUB), product of the ATXN3 gene, ubiquitously expressed in various cell types including peripheral and neuronal tissues and involved in several cellular pathways. Importantly, the expansion of the CAG trinucleotides within the ATXN3 gene leads to an expanded polyglutamine domain in the encoded protein, which has been associated with the onset of the spinocerebellar ataxia type 3, also known as Machado-Joseph disease, the most common dominantly inherited ataxia worldwide. ATXN3 has therefore been under intensive investigation for decades. In this review, we summarize the main functions of ATXN3 in proteostasis, DNA repair and transcriptional regulation, as well as the emerging role in regulating chromatin structure. The mentioned molecular functions of ATXN3 are also reviewed in the context of the pathological expanded form of ATXN3.
Keywords: ATXN3; DNA damage response; chromatin organization; neurodegeneration; polyglutamine neurodegenerative disorders; proteostasis; spinocerebellar ataxia type-3; ubiquitin hydrolase; ubiquitin proteasome system.
Figures


References
-
- Ichikawa Y et al. (2001) The genomic structure and expression of MJD, the Machado-Joseph disease gene. Journal of Human Genetics 46, 413–422. - PubMed
-
- Bettencourt C et al. (2013) Transcript diversity of Machado-Joseph disease gene (ATXN3) is not directly determined by SNPs in exonic or flanking intronic regions. Journal of Molecular Neuroscience 49, 539–543. - PubMed
-
- Bettencourt C et al. (2010) Increased transcript diversity: novel splicing variants of Machado-Joseph Disease gene (ATXN3). Neurogenetics 11, 193–202. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

