Fever Prevention in Patients With Acute Vascular Brain Injury: The INTREPID Randomized Clinical Trial
- PMID: 39320879
- PMCID: PMC11425189
- DOI: 10.1001/jama.2024.14745
Fever Prevention in Patients With Acute Vascular Brain Injury: The INTREPID Randomized Clinical Trial
Abstract
Importance: Fever is associated with worse outcomes in patients with stroke, but whether preventing fever improves outcomes is unclear.
Objective: To determine whether fever prevention after acute vascular brain injury is achievable and impacts functional outcome.
Design, setting, and participants: Open-label randomized clinical trial with blinded outcome assessment that enrolled 686 of 1176 planned critically ill patients with stroke at 43 intensive care units in 7 countries from March 2017 to April 2021 (last date of follow-up was May 12, 2022).
Intervention: Patients randomized to fever prevention (n = 339) were targeted to 37.0 °C for 14 days or intensive care unit discharge using an automated surface temperature management device. Standard care patients (n = 338) received standardized tiered fever treatment on occurrence of temperature of 38 °C or greater.
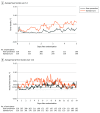
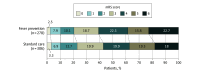
Main outcomes and measures: Primary outcome was daily mean fever burden: the area under the temperature curve above 37.9 °C (total fever burden) divided by the total number of hours in the acute phase, multiplied by 24 hours (°C-hour). The principal secondary outcome was 3-month functional recovery by shift analysis of the 6-category modified Rankin Scale, which is scored from 0 (no symptoms) to 6 (death). Major adverse events included death, pneumonia, sepsis, and malignant cerebral edema.
Results: Enrollment was stopped after a planned interim analysis demonstrated futility of the principal secondary end point. In total, 686 patients were enrolled, and 9 were consented but not randomized, leaving a primary analysis population of 677 patients (254 ischemic stroke, 223 intracerebral hemorrhage, 200 subarachnoid hemorrhage; 345 were female [51%]; median age, 62 years) with 433 (64%) completing the study through 12 months. Daily mean (SD) fever burden was significantly lower in the fever prevention group (0.37 [1.0] °C-hour; range, 0.0-8.0 °C-hour) compared with the standard care group (0.73 [1.1] °C-hour; range, 0.0-10.3 °C-hour) (difference, -0.35 [95% CI, -0.51 to -0.20]; P < .001). Between-group differences for the primary outcome by stroke subtype were -0.10 (95% CI, -0.35 to 0.15) for ischemic stroke, -0.50 (95% CI, -0.78 to -0.22) for intracerebral hemorrhage, and -0.52 (95% CI, -0.81 to -0.23) for subarachnoid hemorrhage (all P < .001 by Wilcoxon rank-sum test). There was no significant difference in functional recovery at 3 months (median modified Rankin Scale score, 4.0 vs 4.0, respectively; odds ratio for a favorable shift in functional outcome, 1.09 [95% CI, 0.81 to 1.46]; P = .54). Major adverse events occurred in 82.2% of participants in the fever prevention group vs 75.9% in the standard care group, including 33.8% vs 34.5% for infections, 14.5% vs 14.0% for cardiac disorders, and 24.5% vs 20.5% for respiratory disorders.
Conclusions and relevance: In patients with acute vascular brain injury, preventive normothermia using an automated surface temperature management device effectively reduced fever burden but did not improve functional outcomes.
Trial registration: ClinicalTrials.gov Identifier: NCT02996266.
Conflict of interest statement
Figures

Comment in
-
Feasibility of Fever Prevention in Vascular Brain Injury.JAMA. 2024 Nov 12;332(18):1521-1522. doi: 10.1001/jama.2024.16415. JAMA. 2024. PMID: 39320871 No abstract available.
References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

