Efficacy and Safety of Crisugabalin (HSK16149) in Adults with Postherpetic Neuralgia: A Phase 3 Randomized Clinical Trial
- PMID: 39320907
- PMCID: PMC11581609
- DOI: 10.1001/jamadermatol.2024.3410
Efficacy and Safety of Crisugabalin (HSK16149) in Adults with Postherpetic Neuralgia: A Phase 3 Randomized Clinical Trial
Abstract
Importance: China carries a heavy burden of postherpetic neuralgia, with an unmet need for novel drugs with greater efficacy and less prominent neurotoxic effects than existing calcium channel ligands.
Objective: To investigate the efficacy and safety of crisugabalin, an oral calcium channel α2δ-1 subunit ligand, for postherpetic neuralgia.
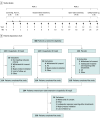
Design, setting, and participants: This randomized clinical trial, carried out between November 9, 2021, and January 5, 2023, at 48 tertiary care centers across China had 2 parts. Part 1 was a phase 3, multicenter, randomized, double-blind, placebo-controlled, parallel-group study consisting of a 2-week screening period, a 7-day run-in period, and a 12-week double-blind treatment period. Part 2 was a 14-week open-label extension study. Investigators, statisticians, trial clinicians, and patients were blinded to trial group assignments. Participants included adults with postherpetic neuralgia with an average daily pain score (ADPS) of at least 4 on the 11-point Numeric Pain Rating Scale over the preceding week, with the exclusion of patients with pain not controlled by prior therapy with pregabalin (≥300 mg/d) or gabapentin (≥1200 mg/d).
Interventions: Patients were randomized 1:1:1 to receive crisugabalin, 20 mg twice daily (ie, 40 mg/d), and crisugabalin, 40 mg twice daily (ie, 80 mg/d), or placebo for 12 weeks. Eligible patients received crisugabalin, 40 mg, twice daily during extension.
Main outcome and measure: The primary efficacy end point was the change from baseline in ADPS at week 12.
Results: The study enrolled 366 patients (121 patients receiving crisugabalin, 40 mg/d; 121 patients receiving crisugabalin, 80 mg/d; 124 patients receiving placebo; median [IQR] age, 63.0 [56.0-69.0] years; 193 men [52.7%]). At week 12, the least squares mean (SD) change from baseline in ADPS was -2.2 (0.2) for crisugabalin, 40 mg/d, and -2.6 (0.2) for crisugabalin, 80 mg/d, vs -1.1 (0.2) for placebo, with a least squares mean difference of -1.1 (95% CI, -1.6 to -0.7; P < .001) and -1.5 (-95% CI, -2.0 to -1.0; P < .001) vs placebo, respectively. No new safety concerns emerged.
Conclusions and relevance: Crisugabalin, 40 mg/d, or crisugabalin, 80 mg/d, was well tolerated and demonstrated a statistically significant improvement in ADPS over placebo.
Trial registration: ClinicalTrials.gov Identifier: NCT05140863.
Conflict of interest statement
Figures


References
-
- Mizukami A, Sato K, Adachi K, et al. Impact of herpes zoster and post-herpetic neuralgia on health-related quality of life in Japanese adults aged 60 years or older: results from a prospective, observational cohort study. Clin Drug Investig. 2018;38(1):29-37. doi: 10.1007/s40261-017-0581-5 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

