Paracrine FGF1 signaling directs pituitary architecture and size
- PMID: 39320918
- PMCID: PMC11459159
- DOI: 10.1073/pnas.2410269121
Paracrine FGF1 signaling directs pituitary architecture and size
Abstract
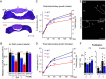
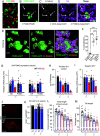
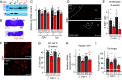
Organ architecture is established during development through intricate cell-cell communication mechanisms, yet the specific signals mediating these communications often remain elusive. Here, we used the anterior pituitary gland that harbors different interdigitated hormone-secreting homotypic cell networks to dissect cell-cell communication mechanisms operating during late development. We show that blocking differentiation of corticotrope cells leads to pituitary hypoplasia with a major effect on somatotrope cells that directly contact corticotropes. Gene knockout of the corticotrope-restricted transcription factor Tpit results in fewer somatotropes, with less secretory granules and a loss of cell polarity, resulting in systemic growth retardation. Single-cell transcriptomic analyses identified FGF1 as a corticotrope-specific Tpit dosage-dependent target gene responsible for these phenotypes. Consistently, genetic ablation of FGF1 in mice phenocopies pituitary hypoplasia and growth impairment observed in Tpit-deficient mice. These findings reveal FGF1 produced by the corticotrope cell network as an essential paracrine signaling molecule participating in pituitary architecture and size.
Keywords: FGF1; GH deficit; cell networks; cell–cell interactions; organogenesis.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures




References
-
- Drouin J., Brière J., “Chap 1–Pituitary development” in The Pituitary, Melmed S., Ed. (Elsevier-Academic Press Publishers, ed. 5, 2022), pp. 1–24.
-
- Chen J., et al. , The adult pituitary contains a cell population displaying stem/progenitor cell and early embryonic characteristics. Endocrinology 146, 3985–3998 (2005). - PubMed
-
- Chen J., et al. , Pituitary progenitor cells tracked down by side population dissection. Stem Cells 27, 1182–1195 (2009). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

