Intravenous Lidocaine for Refractory Pain in Patients With Pancreatic Ductal Adenocarcinoma and Chronic Pancreatitis: A Multicenter Prospective Nonrandomized Pilot Study
- PMID: 39320960
- PMCID: PMC11421722
- DOI: 10.14309/ctg.0000000000000760
Intravenous Lidocaine for Refractory Pain in Patients With Pancreatic Ductal Adenocarcinoma and Chronic Pancreatitis: A Multicenter Prospective Nonrandomized Pilot Study
Abstract
Introduction: Refractory pain is a major clinical problem in patients with pancreatic ductal adenocarcinoma (PDAC) and chronic pancreatitis (CP). New, effective therapies to reduce pain are urgently needed. Intravenous lidocaine is used in clinical practice in patients with PDAC and CP, but its efficacy has not been studied prospectively.
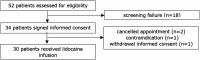
Methods: Multicenter prospective nonrandomized pilot study included patients with moderate or severe pain (Numeric Rating Scale ≥ 4) associated with PDAC or CP in 5 Dutch centers. An intravenous lidocaine bolus of 1.5 mg/kg was followed by continuous infusion at 1.5 mg/kg/hr. The dose was raised every 15 minutes until treatment response (up to a maximum 2 mg/kg/hr) and consecutively administered for 2 hours. Primary outcome was the mean difference in pain severity, preinfusion, and the first day after (Brief Pain Inventory [BPI] scale 1-10). A BPI decrease ≥1.3 points was considered clinically relevant.
Results: Overall, 30 patients were included, 19 with PDAC (63%) and 11 with CP (37%). The mean difference in BPI at day 1 was 1.1 (SD ± 1.3) points for patients with PDAC and 0.5 (SD ± 1.7) for patients with CP. A clinically relevant decrease in BPI on day 1 was reported in 9 of 29 patients (31%), and this response lasted up to 1 month. No serious complications were reported, and only 3 minor complications (vertigo, nausea, and tingling of mouth). Treatment with lidocaine did not impact quality of life.
Discussion: Intravenous lidocaine in patients with painful PDAC and CP did not show an overall clinically relevant reduction of pain. However, this pilot study shows that the treatment is feasible in this patient group and had a positive effect in a third of patients which lasted up to a month with only minor side effects. To prove or exclude the efficacy of intravenous lidocaine, the study should be performed in a study with a greater sample size and less heterogeneous patient group.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of The American College of Gastroenterology.
Conflict of interest statement
Figures


References
-
- Yan BM, Myers RP. Neurolytic celiac plexus block for pain control in unresectable pancreatic cancer. Am J Gastroenterol 2007;102(2):430–8. - PubMed
-
- Damm M, Weniger M, Kölsch AK, et al. The quality of pain management in pancreatic cancer: A prospective multi-center study. Pancreatology 2020;20(7):1511–8. - PubMed
-
- Ammann RW, Muellhaupt B. The natural history of pain in alcoholic chronic pancreatitis. Gastroenterology 1999;116(5):1132–40. - PubMed
-
- Gardner TB, Kennedy AT, Gelrud A, et al. Chronic pancreatitis and its effect on employment and health care experience: Results of a prospective American multicenter study. Pancreas 2010;39(4):498–501. - PubMed
-
- Cohen SM, Kent TS. Etiology, diagnosis, and modern management of chronic pancreatitis: A systematic review. JAMA Surg 2023;158(6):652–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

