B cells drive neuropathic pain-related behaviors in mice through IgG-Fc gamma receptor signaling
- PMID: 39321269
- PMCID: PMC11479571
- DOI: 10.1126/scitranslmed.adj1277
B cells drive neuropathic pain-related behaviors in mice through IgG-Fc gamma receptor signaling
Abstract
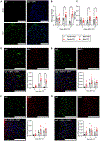
Neuroimmune interactions are essential for the development of neuropathic pain, yet the contributions of distinct immune cell populations have not been fully unraveled. Here, we demonstrate the critical role of B cells in promoting mechanical hypersensitivity (allodynia) after peripheral nerve injury in male and female mice. Depletion of B cells with a single injection of anti-CD20 monoclonal antibody at the time of injury prevented the development of allodynia. B cell-deficient (muMT) mice were similarly spared from allodynia. Nerve injury was associated with increased immunoglobulin G (IgG) accumulation in ipsilateral lumbar dorsal root ganglia (DRGs) and dorsal spinal cords. IgG was colocalized with sensory neurons and macrophages in DRGs and microglia in spinal cords. IgG also accumulated in DRG samples from human donors with chronic pain, colocalizing with a marker for macrophages and satellite glia. RNA sequencing revealed a B cell population in naive mouse and human DRGs. A B cell transcriptional signature was enriched in DRGs from human donors with neuropathic pain. Passive transfer of IgG from injured mice induced allodynia in injured muMT recipient mice. The pronociceptive effects of IgG are likely mediated through immune complexes interacting with Fc gamma receptors (FcγRs) expressed by sensory neurons, microglia, and macrophages, given that both mechanical allodynia and hyperexcitability of dissociated DRG neurons were abolished in nerve-injured FcγR-deficient mice. Consistently, the pronociceptive effects of IgG passive transfer were lost in FcγR-deficient mice. These data reveal that a B cell-IgG-FcγR axis is required for the development of neuropathic pain in mice.
Figures




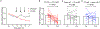

References
-
- van Hecke O, Austin SK, Khan RA, Smith BH, Torrance N, Neuropathic pain in the general population: A systematic review of epidemiological studies. Pain 155, 654–662 (2014). - PubMed
-
- Finnerup NB, Kuner R, Jensen TS, Neuropathic pain: From mechanisms to treatment. Physiol. Rev 101, 259–301 (2021). - PubMed
-
- McMahon SB, La Russa F, Bennett DL, Crosstalk between the nociceptive and immune systems in host defence and disease. Nat. Rev. Neurosci 16, 389–402 (2015). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

