Kappa opioids inhibit spinal output neurons to suppress itch
- PMID: 39321286
- PMCID: PMC11423883
- DOI: 10.1126/sciadv.adp6038
Kappa opioids inhibit spinal output neurons to suppress itch
Abstract
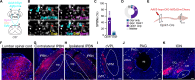
Itch is a protective sensation that drives scratching. Although specific cell types have been proposed to underlie itch, the neural basis for itch remains unclear. Here, we used two-photon Ca2+ imaging of the dorsal horn to visualize neuronal populations that are activated by itch-inducing agents. We identify a convergent population of spinal interneurons recruited by diverse itch-causing stimuli that represents a subset of neurons that express the gastrin-releasing peptide receptor (GRPR). Moreover, we find that itch is conveyed to the brain via GRPR-expressing spinal output neurons that target the lateral parabrachial nuclei. We then show that the kappa opioid receptor agonist nalfurafine relieves itch by selectively inhibiting GRPR spinoparabrachial neurons. These experiments provide a population-level view of the spinal neurons that respond to pruritic stimuli, pinpoint the output neurons that convey itch to the brain, and identify the cellular target of kappa opioid receptor agonists for the inhibition of itch.
Figures






References
-
- Sun Y. G., Chen Z.-F., A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature 448, 700–703 (2007). - PubMed
-
- Kardon A. P., Polgár E., Hachisuka J., Snyder L. M., Cameron D., Savage S., Cai X., Karnup S., Fan C. R., Hemenway G. M., Bernard C. S., Schwartz E. S., Nagase H., Schwarzer C., Watanabe M., Furuta T., Kaneko T., Koerber H. R., Todd A. J., Ross S. E., Dynorphin acts as a neuromodulator to inhibit itch in the dorsal horn of the spinal cord. Neuron 82, 573–586 (2014). - PMC - PubMed
-
- Huang J., Polgár E., Solinski H. J., Mishra S. K., P.-Y. Tseng, Iwagaki N., Boyle K. A., Dickie A. C., Kriegbaum M. C., Wildner H., Zeilhofer H. U., Watanabe M., Riddell J. S., Todd A. J., Hoon M. A., Circuit dissection of the role of somatostatin in itch and pain. Nat. Neurosci. 21, 707–716 (2018). - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

