Skewed epithelial cell differentiation and premature aging of the thymus in the absence of vitamin D signaling
- PMID: 39321290
- PMCID: PMC11423877
- DOI: 10.1126/sciadv.adm9582
Skewed epithelial cell differentiation and premature aging of the thymus in the absence of vitamin D signaling
Abstract
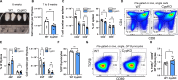
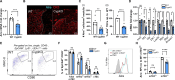
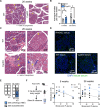
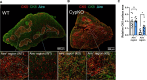
Central tolerance of thymocytes to self-antigen depends on the medullary thymic epithelial cell (mTEC) transcription factor autoimmune regulator (Aire), which drives tissue-restricted antigen (TRA) gene expression. Vitamin D signaling regulates Aire and TRA expression in mTECs, providing a basis for links between vitamin D deficiency and autoimmunity. We find that mice lacking Cyp27b1, which cannot produce hormonally active vitamin D, display profoundly reduced thymic cellularity, with a reduced proportion of Aire+ mTECs, attenuated TRA expression, and poorly defined cortical-medullary boundaries. Markers of T cell negative selection are diminished, and organ-specific autoantibodies are present in knockout (KO) mice. Single-cell RNA sequencing revealed that loss of Cyp27b1 skews mTEC differentiation toward Ccl21+ intertypical TECs and generates a gene expression profile consistent with premature aging. KO thymi display accelerated involution and reduced expression of thymic longevity factors. Thus, loss of thymic vitamin D signaling disrupts normal mTEC differentiation and function and accelerates thymic aging.
Figures



References
-
- Kadouri N., Nevo S., Goldfarb Y., Abramson J., Thymic epithelial cell heterogeneity: TEC by TEC. Nat. Rev. Immunol. 20, 239–253 (2020). - PubMed
-
- Wu H., Qin X., Dai H., Zhang Y., Time-course transcriptome analysis of medullary thymic epithelial cells in the early phase of thymic involution. Mol. Immunol. 99, 87–94 (2018). - PubMed
-
- Bornstein C., Nevo S., Giladi A., Kadouri N., Pouzolles M., Gerbe F., David E., Machado A., Chuprin A., Toth B., Goldberg O., Itzkovitz S., Taylor N., Jay P., Zimmermann V. S., Abramson J., Amit I., Single-cell mapping of the thymic stroma identifies IL-25-producing tuft epithelial cells. Nature 559, 622–626 (2018). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

