Preserving surface strain in nanocatalysts via morphology control
- PMID: 39321292
- PMCID: PMC11423881
- DOI: 10.1126/sciadv.adp3788
Preserving surface strain in nanocatalysts via morphology control
Abstract
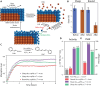
Engineering strain critically affects the properties of materials and has extensive applications in semiconductors and quantum systems. However, the deployment of strain-engineered nanocatalysts faces challenges, in particular in maintaining highly strained nanocrystals under reaction conditions. Here, we introduce a morphology-dependent effect that stabilizes surface strain even under harsh reaction conditions. Using four-dimensional scanning transmission electron microscopy (4D-STEM), we found that cube-shaped core-shell Au@Pd nanoparticles with sharp-edged morphologies sustain coherent heteroepitaxial interfaces with larger critical thicknesses than morphologies with rounded edges. This configuration inhibits dislocation nucleation due to reduced shear stress at corners, as indicated by molecular dynamics simulations. A Suzuki-type cross-coupling reaction shows that our approach achieves a fourfold increase in activity over conventional nanocatalysts, owing to the enhanced stability of surface strain. These findings contribute to advancing the development of advanced nanocatalysts and indicate broader applications for strain engineering in various fields.
Figures



References
-
- Debe M. K., Electrocatalyst approaches and challenges for automotive fuel cells. Nature 486, 43–51 (2012). - PubMed
-
- Wang H., Xu S., Tsai C., Li Y., Liu C., Zhao J., Liu Y., Yuan H., Abild-Pedersen F., Prinz F. B., Nørskov J. K., Cui Y., Direct and continuous strain control of catalysts with tunable battery electrode materials. Science 354, 1031–1036 (2016). - PubMed
-
- Luo M., Guo S., Strain-controlled electrocatalysis on multimetallic nanomaterials. Nat. Rev. Mater. 2, 17059 (2017).
-
- Bu L., Zhang N., Guo S., Zhang X., Li J., Yao J., Wu T., Lu G., Ma J.-Y., Su D., Huang X., Biaxially strained PtPb/Pt core/shell nanoplate boosts oxygen reduction catalysis. Science 354, 1410–1414 (2016). - PubMed
-
- He T., Wang W., Shi F., Yang X., Li X., Wu J., Yin Y., Jin M., Mastering the surface strain of platinum catalysts for efficient electrocatalysis. Nature 598, 76–81 (2021). - PubMed
LinkOut - more resources
Full Text Sources

