Comprehensive Analysis of Paraneoplastic Neurologic Syndrome and PNS-CARE Diagnostic Criteria in Clinical Practice
- PMID: 39321395
- PMCID: PMC11443324
- DOI: 10.1212/NXI.0000000000200316
Comprehensive Analysis of Paraneoplastic Neurologic Syndrome and PNS-CARE Diagnostic Criteria in Clinical Practice
Abstract
Background and objectives: Paraneoplastic neurologic syndrome (PNS) diagnostic criteria were first proposed in 2004 and updated in 2021. The PNS-CARE score, derived from the updated criteria, is a composite model for assigning likelihood for patients with suspected PNS. In this study, we evaluated the utility and applicability of the 2021 PNS-CARE score and present our PNS cohort.
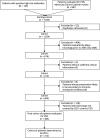
Methods: This is a retrospective study. We identified Mayo Clinic patients suspected to have PNS (1/2005-12/2020) and collected relevant information including demographics, PNS presentation, and clinical outcomes. Inclusion criteria were the following: (1) patients with a syndrome consistent with PNS and (2) patients with sufficient information available in charts. Exclusion criteria were the following: (1) evaluation only before 2005, (2) patients not evaluated by neurology, (3) presentation after immune checkpoint inhibitors, and (4) syndromes not included in 2021 criteria. All patients were evaluated for the 2021 and 2004 PNS criteria.
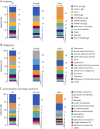
Results: We identified 484 patients suspected to have PNS at initial presentation, of whom 212 (44%) were considered to have PNS after completion of evaluation. Among these 212 patients, the most common autoantibodies were PCA1 (Yo)-IgG (17%), KLHL11-IgG (16%), and CRMP5-IgG (14%) and the most common phenotypes were rapidly progressive cerebellar syndrome (29%), brainstem encephalitis (14%), and limbic encephalitis (8%). The 2021 PNS criteria definite/probable categorization (PNS-CARE score ≥ 6) had a sensitivity and specificity of 93% and 100%, respectively, while the 2004 PNS criteria definite categorization had a sensitivity and specificity of 67% and 99%, respectively. We found 15 patients with a PNS-CARE score ≤5 who likely had PNS on our review. The most common presentation among these patients was KLHL11-IgG brainstem encephalitis (7/15, 47%) with likely burned-out testicular tumor.
Discussion: Our study validates the PNS-CARE score. A clearer understanding of typical PNS presentation and common underlying malignancies and autoantibodies can aid in earlier and more accurate diagnosis, which is crucial for downstream clinical decisions. Some patients with an intermediate-risk phenotype do not meet probable/definite criteria despite the presence of high-risk antibodies and/or underlying malignancy.
Conflict of interest statement
H. Zhao-Fleming, M. Rezk, S. Shah, and P. Gupta report no disclosures; A. Zekeridou reported grants from Roche/Genentech outside the submitted work and had patents for DACH1-IgG, PDE10A-IgG and Tensacin-R-IgG as biomarkers of neurological autoimmunity pending; E.P. Flanagan has served on advisory boards for Alexion, Genentech, Horizon Therapeutics and UCB. He has received research support from UCB. He has received speaker honoraria from Pharmacy Times. He received royalties from UpToDate. E.P. Flanagan is a site principal investigator in a randomized clinical trial of rozanolixizumab for relapsing myelin oligodendrocyte glycoprotein antibody-associated disease run by UCB and a site principal investigator and a member of the steering committee for a clinical trial of satralizumab for relapsing myelin oligodendrocyte glycoprotein antibody-associated disease run by Roche/Genentech. E.P. Flanagan has received funding from the NIH (R01NS113828); is a member of the medical advisory board of the MOG project; and is an editorial board member of
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
