Structural basis of the human transcriptional Mediator regulated by its dissociable kinase module
- PMID: 39321804
- PMCID: PMC11832219
- DOI: 10.1016/j.molcel.2024.09.001
Structural basis of the human transcriptional Mediator regulated by its dissociable kinase module
Abstract
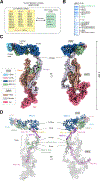
The eukaryotic transcriptional Mediator comprises a large core (cMED) and a dissociable CDK8 kinase module (CKM). cMED recruits RNA polymerase II (RNA Pol II) and promotes pre-initiation complex formation in a manner repressed by the CKM through mechanisms presently unknown. Herein, we report cryoelectron microscopy structures of the complete human Mediator and its CKM. The CKM binds to multiple regions on cMED through both MED12 and MED13, including a large intrinsically disordered region (IDR) in the latter. MED12 and MED13 together anchor the CKM to the cMED hook, positioning CDK8 downstream and proximal to the transcription start site. Notably, the MED13 IDR obstructs the recruitment of RNA Pol II/MED26 onto cMED by direct occlusion of their respective binding sites, leading to functional repression of cMED-dependent transcription. Combined with biochemical and functional analyses, these structures provide a conserved mechanistic framework to explain the basis for CKM-mediated repression of cMED function.
Keywords: CDK8; CKM; CTD; IDR; MED12; MED13; MED26; PIC; RNA polymerase II; mediator; transcription.
Copyright © 2024 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures






Update of
-
Structural basis of the human transcriptional Mediator complex modulated by its dissociable Kinase module.bioRxiv [Preprint]. 2024 Jul 3:2024.07.01.601608. doi: 10.1101/2024.07.01.601608. bioRxiv. 2024. Update in: Mol Cell. 2024 Oct 17;84(20):3932-3949.e10. doi: 10.1016/j.molcel.2024.09.001. PMID: 39005267 Free PMC article. Updated. Preprint.
References
-
- El Khattabi L, Zhao H, Kalchschmidt J, Young N, Jung S, Van Blerkom P, Kieffer-Kwon P, Kieffer-Kwon KR, Park S, Wang X, et al. (2019). A Pliable Mediator Acts as a Functional Rather Than an Architectural Bridge between Promoters and Enhancers. Cell 178, 1145–1158 e1120. 10.1016/j.cell.2019.07.011: . - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

