Stub1 promotes degradation of the activated Diaph3: A negative feedback regulatory mechanism of the actin nucleator
- PMID: 39322015
- PMCID: PMC11736009
- DOI: 10.1016/j.jbc.2024.107813
Stub1 promotes degradation of the activated Diaph3: A negative feedback regulatory mechanism of the actin nucleator
Abstract
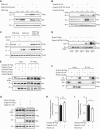
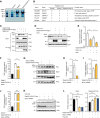
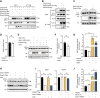
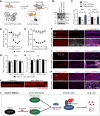
The formin protein Diaph3 is an actin nucleator that regulates numerous cytoskeleton-dependent cellular processes through the activation of actin polymerization. Expression and activity of Diaph3 is tightly regulated: lack of Diaph3 results in developmental defects and embryonic lethality in mice, while overexpression of Diaph3 causes auditory neuropathy. It is known that Diaph3 homophilic interactions include the intramolecular interaction of its Dia-inhibitory domain (DID)-diaphanous autoregulatory domain (DAD) domains and the intermolecular interactions of DD-DD domains or FH2-FH2 domains. However, the physiological significance of these interactions in Diaph3 protein stability and activity is not fully understood. In this study, we show that FH2-FH2 interaction promotes Diaph3 activity, while DID-DAD and DD-DD interactions inhibit Diaph3 activity through distinct mechanisms. DID-DAD interaction is responsible for the autoinhibition of Diaph3 protein, which is disrupted by binding of Rho GTPases. Interestingly, we find that DID-DAD interaction stabilizes the expression of each DID or DAD domain against proteasomal-mediated degradation. Disruption of DID-DAD interaction by RhoA binding or M1041A mutation causes increased Diaph3 activity and accelerated degradation of the activated Diaph3 protein. Further, the activated Diaph3 is ubiquitinated at K1142/1143/1144 lysine residues by the E3 ligase Stub1. Expression of Stub1 is causally related to the stability and activity of Diaph3. Knockdown of Stub1 in mouse cochlea results in hair cell stereocilia defects, neuronal degeneration, and hearing loss, resembling the phenotypes of mice overexpressing Diaph3. Thus, our study reports a novel regulatory mechanism of Diaph3 protein expression and activity whereby the active but not inactive Diaph3 is readily degraded to prevent excessive actin polymerization.
Keywords: Diaph3; Stub1; actin polymerization; autoinhibition; hearing loss; negative feedback; protein stability.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures


Similar articles
-
Negative cooperativity regulates ligand activation of DIAPH1 and other diaphanous related formins.Commun Biol. 2025 May 21;8(1):776. doi: 10.1038/s42003-025-08222-5. Commun Biol. 2025. PMID: 40399622 Free PMC article.
-
Biochemical characterization of the Rho GTPase-regulated actin assembly by diaphanous-related formins, mDia1 and Daam1, in platelets.J Biol Chem. 2008 Mar 28;283(13):8746-55. doi: 10.1074/jbc.M707839200. Epub 2008 Jan 24. J Biol Chem. 2008. PMID: 18218625
-
Regulation of INF2-mediated actin polymerization through site-specific lysine acetylation of actin itself.Proc Natl Acad Sci U S A. 2020 Jan 7;117(1):439-447. doi: 10.1073/pnas.1914072117. Epub 2019 Dec 23. Proc Natl Acad Sci U S A. 2020. PMID: 31871199 Free PMC article.
-
Role of Cytoskeletal Diaphanous-Related Formins in Hearing Loss.Cells. 2022 May 24;11(11):1726. doi: 10.3390/cells11111726. Cells. 2022. PMID: 35681420 Free PMC article. Review.
-
Formin proteins: a domain-based approach.Trends Biochem Sci. 2005 Jun;30(6):342-53. doi: 10.1016/j.tibs.2005.04.014. Trends Biochem Sci. 2005. PMID: 15950879 Review.
References
-
- Evangelista M., Pruyne D., Amberg D.C., Boone C., Bretscher A. Formins direct Arp2/3-independent actin filament assembly to polarize cell growth in yeast. Nat. Cell Biol. 2002;4:260–269. - PubMed
-
- Feierbach B., Chang F. Roles of the fission yeast formin for3p in cell polarity, actin cable formation and symmetric cell division. Curr. Biol. 2001;11:1656–1665. - PubMed
-
- Severson A.F., Baillie D.L., Bowerman B. A Formin Homology protein and a profilin are required for cytokinesis and Arp2/3-independent assembly of cortical microfilaments in C. elegans. Curr. Biol. 2002;12:2066–2075. - PubMed
-
- Miralles F., Posern G., Zaromytidou A.I., Treisman R. Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell. 2003;113:329–342. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

