Stereocontrolled Synthesis of Chiral Helicene-Indenido ansa- and Half-Sandwich Metal Complexes and Their Use in Catalysis
- PMID: 39322620
- PMCID: PMC11701364
- DOI: 10.1002/anie.202414698
Stereocontrolled Synthesis of Chiral Helicene-Indenido ansa- and Half-Sandwich Metal Complexes and Their Use in Catalysis
Abstract
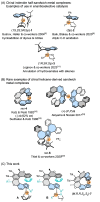
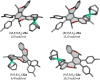
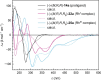
Despite recent tremendous progress in the synthesis of nonplanar chiral aromatics, and helicenes in particular, their conversion to half-sandwich or sandwich transition metal complexes still lags behind, although they represent an attractive family of modular and underexplored chiral architectures with a potential catalytic use. In this work, starting from various chiral helicene-indene proligands, we prepared the enantio- and diastereopure oxa[6]- and oxa[7]helicene-indenido half-sandwich RhI and RhIII complexes and oxa[7]helicene-bisindenido ansa-metallocene FeII complex. To document their use, oxahelicene-indenido half-sandwich RhIII complexes were employed as chiral catalysts in enantioselective C-H arylation of benzo[h]quinolines with 1-diazonaphthoquinones to afford a series of axially chiral biaryls in mostly good to high yields and in up to 96 : 4 er. Thus, we developed stereocontrolled synthesis of chiral helicene-indenido ansa- and half-sandwich metal complexes, successfully demonstrated the first use of such helicene Cp-related metal complexes in enantioselective catalysis, and described an unusual sequence of efficient central-to-helical-to-planar-to-axial chirality transfer.
Keywords: C−H activation; ansa-metallocene; enantioselective catalysis; half-sandwich complexes; helicenes.
© 2024 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Pammer F., Thiel W. R., Coord. Chem. Rev. 2014, 270–271, 14–30.
-
- Schumann H., Stenzel O., Dechert S., Girgsdies F., Blum J., Gelman D., Halterman R. L., Eur. J. Inorg. Chem. 2002, 2002, 211–219.
-
- Gutnov A., Heller B., Fischer C., Drexler H.-J., Spannenberg A., Sundermann B., Sundermann C., Angew. Chem. Int. Ed. 2004, 43, 3795–3797. - PubMed
-
- Heller B., Gutnov A., Fischer C., Drexler H.-J., Spannenberg A., Redkin D., Sundermann C., Sundermann B., Chem. Eur. J. 2007, 13, 1117–1128. - PubMed
-
- Hapke M., Kral K., Fischer C., Spannenberg A., Gutnov A., Redkin D., Heller B., J. Org. Chem. 2010, 75, 3993–4003. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

