Pola-R-CHP or R-CHOEP for first-line therapy of younger patients with high-risk diffuse large B-cell lymphoma: a retrospective comparison of two randomized phase 3 trials
- PMID: 39322715
- PMCID: PMC11588652
- DOI: 10.1038/s41375-024-02420-6
Pola-R-CHP or R-CHOEP for first-line therapy of younger patients with high-risk diffuse large B-cell lymphoma: a retrospective comparison of two randomized phase 3 trials
Conflict of interest statement
Competing interests: GL received research grants not related to this manuscript from AGIOS, AQUINOX, AstraZeneca, Bayer, Celgene, Gilead, Janssen, MorphoSys, Novartis, F. Hoffmann-La Roche Ltd, and Verastem. GL received honoraria from ADC Therapeutics, AbbVie, Amgen, AstraZeneca, Bayer, BeiGene, BMS, Celgene, Constellation, Genase, Genmab, Gilead, Hexal/Sandoz, Immagene, Incyte, Janssen, Karyopharm, Lilly, Miltenyi, MorphoSys, MSD, NanoString, Novartis, PentixaPharm, Pierre Fabre, F. Hoffmann-La Roche Ltd, and Sobi.
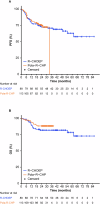
Figures
References
-
- Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–42. - PubMed
-
- Cunningham D, Hawkes EA, Jack A, Qian W, Smith P, Mouncey P, et al. Rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone in patients with newly diagnosed diffuse large B-cell non-Hodgkin lymphoma: a phase 3 comparison of dose intensification with 14-day versus 21-day cycles. Lancet. 2013;381:1817–26. - PubMed
-
- Pfreundschuh M, Poeschel V, Zeynalova S, Hanel M, Held G, Schmitz N, et al. Optimization of rituximab for the treatment of diffuse large B-cell lymphoma (II): extended rituximab exposure time in the SMARTE-R-CHOP-14 trial of the German high-grade non-Hodgkin lymphoma study group. J Clin Oncol. 2014;32:4127–33. - PubMed
-
- Friedrichs B, Nickelsen M, Ziepert M, Altmann B, Haenel M, Viardot A, et al. Doubling rituximab in high-risk patients with aggressive B-cell lymphoma -results of the DENSE-R-MegaCHOEP trial. Brit J Haematol. 2019;184:760–68. - PubMed
-
- Lugtenburg PJ, de Nully Brown P, van der Holt B, D’Amore FA, Koene HR, de Jongh E, et al. Rituximab-CHOP with early rituximab intensification for diffuse large B-cell lymphoma: a randomized phase III trial of the HOVON and the nordic lymphoma group (HOVON-84). J Clin Oncol. 2020;38:3377–87. - PubMed
LinkOut - more resources
Full Text Sources

