RIG-I is an intracellular checkpoint that limits CD8+ T-cell antitumour immunity
- PMID: 39322862
- PMCID: PMC11555380
- DOI: 10.1038/s44321-024-00136-9
RIG-I is an intracellular checkpoint that limits CD8+ T-cell antitumour immunity
Abstract
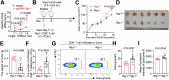
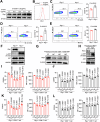
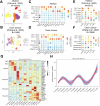
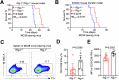
Retinoic acid-inducible gene I (RIG-I) is a pattern recognition receptor involved in innate immunity, but its role in adaptive immunity, specifically in the context of CD8+ T-cell antitumour immunity, remains unclear. Here, we demonstrate that RIG-I is upregulated in tumour-infiltrating CD8+ T cells, where it functions as an intracellular checkpoint to negatively regulate CD8+ T-cell function and limit antitumour immunity. Mechanistically, the upregulation of RIG-I in CD8+ T cells is induced by activated T cells, and directly inhibits the AKT/glycolysis signalling pathway. In addition, knocking out RIG-I enhances the efficacy of adoptively transferred T cells against solid tumours, and inhibiting RIG-I enhances the response to PD-1 blockade. Overall, our study identifies RIG-I as an intracellular checkpoint and a potential target for alleviating inhibitory constraints on T cells in cancer immunotherapy, either alone or in combination with an immune checkpoint inhibitor.
Keywords: AKT/Glycolysis Signalling Pathway; CD8+ T cells; Immune Checkpoint; Immunotherapy; RIG-I.
© 2024. The Author(s).
Conflict of interest statement
Figures






References
-
- Chen H, Duan X, Deng X, Huang Y, Zhou X, Zhang S, Zhang X, Liu P, Yang C, Liu G et al (2023) EBV-upregulated B7-H3 inhibits NK cell-mediated antitumor function and contributes to nasopharyngeal carcinoma progression. Cancer Immunol Res 11:830–846 - PubMed
-
- Curigliano G, Gelderblom H, Mach N, Doi T, Tai D, Forde PM, Sarantopoulos J, Bedard PL, Lin CC, Hodi FS et al (2021) Phase I/Ib clinical trial of sabatolimab, an anti-TIM-3 antibody, alone and in combination with spartalizumab, an anti-PD-1 antibody, in advanced solid tumors. Clin Cancer Res 27:3620–3629 - PubMed
MeSH terms
Substances
Grants and funding
- 82230067/MOST | National Natural Science Foundation of China (NSFC)
- 82103433/MOST | National Natural Science Foundation of China (NSFC)
- 8204415/MOST | National Natural Science Foundation of China (NSFC)
- 32200936/MOST | National Natural Science Foundation of China (NSFC)
- 82103306/MOST | National Natural Science Foundation of China (NSFC)
- 82304589/MOST | National Natural Science Foundation of China (NSFC)
- 32470961/MOST | National Natural Science Foundation of China (NSFC)
- 2021B1212040004/Guangdong Provincial Key Laboratory of Construction Foundation ()
- 2024A1515012563/GDSTC | Basic and Applied Basic Research Foundation of Guangdong Province ()
- 2021A1515220187/GDSTC | Basic and Applied Basic Research Foundation of Guangdong Province ()
- 2021A1515110561/GDSTC | Basic and Applied Basic Research Foundation of Guangdong Province ()
- 2020A1515110013/GDSTC | Basic and Applied Basic Research Foundation of Guangdong Province ()
- JCYJ20220818101808018/Shenzhen Municipal Science and Technology Innovation Council | Shenzhen Science and Technology Innovation Program ()
- 2021KYQD-04/Scientific Research start up Project of Zhuhai People's Hospital
- SL2024A03J00598/the Science and Technology Planning Project of Guangzhou
LinkOut - more resources
Full Text Sources
Research Materials

