Structure and Dynamics of ATP and the ATP-Zn2+ Complex in Solution
- PMID: 39323317
- PMCID: PMC11457213
- DOI: 10.1021/acs.jpclett.4c02296
Structure and Dynamics of ATP and the ATP-Zn2+ Complex in Solution
Abstract
Despite the crucial role of ATP in life and artificial life-like applications, fundamental aspects relevant to its function, such as its conformational properties and its interaction with water and ions, remain unclear. Here, by integrating linear and two-dimensional infrared spectroscopy with ab initio molecular dynamics, we provide a detailed characterization of the vibrational spectra of the phosphate groups in ATP and in its complex with Zn2+ in water. Our study highlights the role of conformational disorder and solvation dynamics, beyond the harmonic normal-mode analysis, and reveals a complex scenario in which electronic and environmental effects tune the coupling between phosphate vibrations. We identify βγ-bidentate and αβγ-tridentate modes as the preferential coordination modes of Zn2+, as was proposed in the literature for Mg2+, although this conclusion is reached by a different spectral interpretation.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
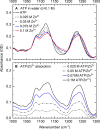
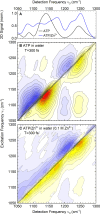

 and
and  (yellow),
(yellow),  (light blue), and
(light blue), and  (green).
(green).
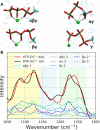
Similar articles
-
Infrared Spectroscopy of Li+ Solvation in Diglyme: Ab Initio Molecular Dynamics and Experiment.J Phys Chem B. 2023 Oct 26;127(42):9191-9203. doi: 10.1021/acs.jpcb.3c05612. Epub 2023 Oct 11. J Phys Chem B. 2023. PMID: 37820068 Free PMC article.
-
Two-dimensional infrared spectroscopy of intermolecular hydrogen bonds in the condensed phase.Acc Chem Res. 2009 Sep 15;42(9):1220-8. doi: 10.1021/ar900006u. Acc Chem Res. 2009. PMID: 19425543 Review.
-
Ab Initio Molecular Dynamics Study of Aqueous Solutions of Magnesium and Calcium Nitrates: Hydration Shell Structure, Dynamics and Vibrational Echo Spectroscopy.J Phys Chem B. 2022 Jan 20;126(2):528-544. doi: 10.1021/acs.jpcb.1c08545. Epub 2022 Jan 8. J Phys Chem B. 2022. PMID: 35001626
-
Quantum effects on vibrational and electronic spectra of hydrazine studied by "on-the-fly" ab initio ring polymer molecular dynamics.J Phys Chem A. 2009 Mar 12;113(10):1985-94. doi: 10.1021/jp8081936. J Phys Chem A. 2009. PMID: 19199678
-
Interface-specific ultrafast two-dimensional vibrational spectroscopy.Acc Chem Res. 2009 Sep 15;42(9):1332-42. doi: 10.1021/ar900016c. Acc Chem Res. 2009. PMID: 19441810 Review.
References
LinkOut - more resources
Full Text Sources

