Patient and care partner perspectives and preferences related to myasthenia gravis treatment: A qualitative study
- PMID: 39323457
- PMCID: PMC11422664
- DOI: 10.1002/hsr2.70081
Patient and care partner perspectives and preferences related to myasthenia gravis treatment: A qualitative study
Abstract
Background and aims: Due to the high symptom and treatment burden in myasthenia gravis (MG), understanding patient and care partner perspectives and preferences is crucial.
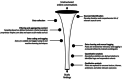
Methods: This study used voice analysis and virtual focus groups to understand patient and care partner experiences with MG-related symptoms, treatments, and preferences. The voice analysis via social media listening used artificial intelligence-powered tools to gather and structure public digital conversations on MG. Focus groups included people living with MG and care partners who completed a questionnaire and participated in a 1-h virtual session facilitated using a semi-structured interview guide. Qualitative data were aggregated, transcribed, and thematically analyzed.
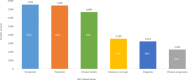
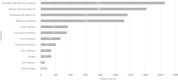
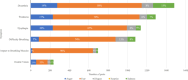
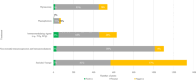
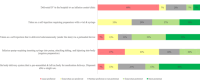
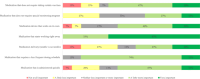
Results: The voice analysis examined 11,554 posts from 8321 individuals, discussing MG symptoms, treatments, and burden. Of 7563 symptom-related posts, 5902 (78%) conveyed negative, 1427 (19%) neutral, and 234 (3%) positive sentiment. The most frequently mentioned symptoms were categorized as dysarthria, muscle weakness, and dysphagia. MG treatment sentiment analysis identified 6667 posts (67%) as neutral, 2887 (29%) as negative, and 350 (4%) as positive. For the focus groups, 15 individuals (12 patients and 3 care partners) completed the questionnaire and 14 participated in the virtual focus group sessions. The 15 participants who completed the questionnaire prioritized treatment convenience, symptom control for improved quality of life, and preventing potential MG crises in their current treatment. New treatment expectations included increased effectiveness, less frequent dosing, faster onset, and fewer side effects. Participants were also receptive to wearable medication delivery systems placed on the body and valued direct involvement in treatment decisions.
Conclusion: Patients and care partners are often negatively impacted by MG symptoms and value convenient and fast-acting treatments that control symptoms with minimal side effects. Considering patient preferences may help optimize treatment decisions and improve patients' overall well-being and satisfaction in their care.
Keywords: myasthenia gravis; patient perspective; patient preference; symptoms; treatment.
© 2024 The Author(s). Health Science Reports published by Wiley Periodicals LLC.
Conflict of interest statement
GG, JP, LJ, LS, and ZC are current employees of Janssen Scientific Affairs, LLC. KLD, MY, and JS are employees of EPI‐Q, Inc., which received funding from Janssen Scientific Affairs, LLC, associated with the development and execution of this study. JF and JR are current or former employees of Inspire, which received funding associated with the development and execution of this study. MT and WP are employees of CorEvitas, which received funding from Janssen Scientific Affairs, LLC, associated with the execution of this study. PN has served consultancy/advisory roles for Argenx, Alexion, Dianthus, GSK, ImmunAbs, Janssen, Novartis, UCB; received research funding from Alexion, Dianthus, Janssen, Patient Centered Outcomes Research Institute (PCORI), UCB; and served on the data safety monitoring board for Sanofi. AF has received honorarium for advisory boards from Argenx, Immunovant and UCB. BW is a Patient Engagement Research Council (PERC) member and has received payment from Janssen Scientific Affairs, LLC. RG has received honoraria for advisory boards from Alexion, Argenx, UCB, Janssen, Alexion and speaking‐related honoraria from Alexion, Argenx, and UCB.
Figures
References
-
- Gilhus NE, Longo DL. Myasthenia gravis. N Engl J Med. 2016;375(26):2570‐2581. - PubMed
LinkOut - more resources
Full Text Sources

