The molecular tumor board as a step in cancer patient management: a southern Italian experience
- PMID: 39323465
- PMCID: PMC11422073
- DOI: 10.3389/fmed.2024.1432628
The molecular tumor board as a step in cancer patient management: a southern Italian experience
Erratum in
-
Corrigendum: The molecular tumor board as a step in cancer patient management: a southern Italian experience.Front Med (Lausanne). 2024 Oct 25;11:1511339. doi: 10.3389/fmed.2024.1511339. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39526241 Free PMC article.
Abstract
Introduction: The management of cancer patients follows a Diagnostic Therapeutic and Care Pathway (PDTA) approach, aimed at achieving the optimal balance between care and quality of life. To support this process, precision medicine and innovative technologies [e.g., next-generation sequencing (NGS)] allow rapid identification of genetic-molecular alterations useful for the design of PDTA-approved therapies. If the standard approach proves inadequate, the Molecular Tumor Board (MTB), a group comprising specialists from diverse disciplines, can step in to evaluate a broader molecular profile, proposing potential therapies beyond evidence levels I-II or considering enrolment in clinical trials. Our aim is to analyze the role of the MTB in the entire management of patients in our institute and its impact on the strategy of personalized medicine, particularly when all approved treatments have failed.
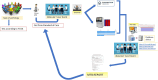
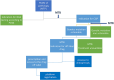
Materials and methods: In alignment with European and national guidelines, a panel of clinicians and preclinical specialists from our institution was defined as the MTB core team. We designed and approved a procedure for the operation of this multidisciplinary group, which is the only one operating in the Puglia region.
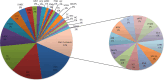
Results and discussion: In 29 months (2021-2023), we discussed and analyzed 93 patients. A total of 44% presented pathogenic alterations, of which 40.4% were potentially actionable. Only 11 patients were proposed for enrollment in clinical trials, treatment with off-label drugs, or AIFA (the Italian pharmaceutical agency for drugs)-5% funding. Our process indicators, time to analysis, and number of patient cases discussed are in line with the median data of other European institutions. Such findings underscore both the importance and usefulness of the integration of an MTB process into the care of oncology patients.
Keywords: Molecular Tumor Board; comprehensive genomic profile; liquid biopsy; precision medicine; team.
Copyright © 2024 Tommasi, Maurmo, Rizzo, Carella, Ranieri, De Summa, Mannavola, Chiurì, Guida, Nisi, Montrone, Giotta, Patruno, Lacalamita, Pilato, Zito, Fucci, Coppola, Ditonno, Nardulli, Quaresmini and Strippoli.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

References
-
- Italian Ministry of Health. Decreto Ministeriale n. 70 2 Aprile 2015. (2015). Available at: https://www.camera.it/temiap/2016/09/23/OCD177-2353.pdf
-
- Mosele F, Remon J, Mateo J, Westphalen CB, Barlesi F, Lolkema MP, et al. . Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: a report from the ESMO precision medicine working group. Ann Oncol. (2020) 31:1491–505. doi: 10.1016/j.annonc.2020.07.014, PMID: - DOI - PubMed
LinkOut - more resources
Full Text Sources

