Iptacopan Efficacy and Safety to Treat Paroxysmal Nocturnal Hemoglobinuria (PNH): A Systematic Review and Meta-Analysis
- PMID: 39323666
- PMCID: PMC11423926
- DOI: 10.7759/cureus.67830
Iptacopan Efficacy and Safety to Treat Paroxysmal Nocturnal Hemoglobinuria (PNH): A Systematic Review and Meta-Analysis
Retraction in
-
Retraction: Iptacopan Efficacy and Safety to Treat Paroxysmal Nocturnal Hemoglobinuria (PNH): A Systematic Review and Meta-Analysis.Cureus. 2025 Jan 22;17(1):r163. doi: 10.7759/cureus.r163. eCollection 2025 Jan. Cureus. 2025. PMID: 39850270 Free PMC article.
Abstract
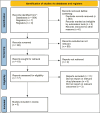
Paroxysmal nocturnal hemoglobinuria (PNH) is an uncommon blood condition caused by complement-mediated hemolysis. In early clinical studies, iptacopan, an oral factor B inhibitor, showed promise in treating PNH. This systematic review aimed to compile information on the effectiveness and safety of iptacopan for PNH. A systematic review was performed using Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) standards. The Medline, Embase, PubMed, and Cochrane Central databases were searched for randomized controlled trials (RCTs) and observational studies that evaluated iptacopan for PNH. The primary efficacy outcomes were hemoglobin and lactate dehydrogenase (LDH) changes. A fixed-effects model was used for the meta-analysis. Six studies (three prospective cohorts, two RCTs, and one non-randomized trial) were included, comprising 197 patients. Iptacopan therapy significantly increased hemoglobin levels (mean differences (MD) = 12.98 g/L; 95% CI: 11.82-14.13; p < 0.0001) and decreased LDH (MD = -83.55 IU/L; 95% CI: -83.77 to -83.34; p < 0.0001). Subgroup analyses revealed more significant hemoglobin increases in European vs. Asian populations, studies with baseline Hb > 10 g/dL vs. < 10 g/dL, and studies lasting > 24 weeks vs. ≤ 24 weeks. LDH decreases were more pronounced in studies with baseline LDH > 500 IU/L vs. < 500 IU/L. The incidence of adverse events ranged from 66% to 90%, with the most common being headache, nasopharyngitis, and diarrhea. Serious adverse events occurred in 0-20% of patients across studies. Only one patient withdrew because adverse effects were reported across all studies. Preliminary data support iptacopan's effectiveness in improving hemoglobin levels and lowering hemolysis in PNH, both as a monotherapy and in combination with usual treatment. The safety profile appears to be excellent, with a minimal incidence of major adverse events and treatment discontinuation. Further studies are needed to validate the effectiveness and safety of larger, longer-term trials. Iptacopan is a viable oral therapy option for PNH.
Keywords: complement inhibition; factor b inhibitor; hemoglobin; hemolysis; iptacopan; lactate dehydrogenase; paroxysmal nocturnal hemoglobinuria.
Copyright © 2024, Dighriri et al.
Conflict of interest statement
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures

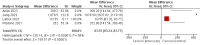







References
-
- Paroxysmal nocturnal hemoglobinuria and the complement system: recent insights and novel anticomplement strategies. Risitano AM. Adv Exp Med Biol. 2013;735:155–172. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
