The Efficacy and Safety of Dexamethasone Intracanalicular Insert Use in Patients with Chronic Seasonal/Perennial Allergic Conjunctivitis: A Systematic Review and Meta-Analysis
- PMID: 39323726
- PMCID: PMC11423825
- DOI: 10.2147/OPTH.S470657
The Efficacy and Safety of Dexamethasone Intracanalicular Insert Use in Patients with Chronic Seasonal/Perennial Allergic Conjunctivitis: A Systematic Review and Meta-Analysis
Abstract
Objective: This meta-analysis evaluated the efficacy and safety of DEXTENZA, an intracanalicular dexamethasone insert, for the treatment of seasonal/perennial allergic conjunctivitis.
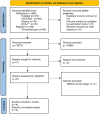
Methods: Multiple databases, including PubMed, the Cochrane Central Register of Controlled Trials (CENTRAL), ClinicalTrials.gov, the Directory of Open Access Journals, and Scopus, were searched for randomized controlled trials (RCTs) comparing the efficacy of DEXTENZA with a placebo. The primary efficacy endpoint was the change in the conjunctival allergen challenge (CAC) model. The GRADE approach was used to assess the certainty of evidence, and the revised Cochrane risk of bias tool for randomized trials was employed to assess bias.
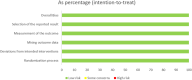
Results: Four RCTs involving 323 participants met the eligibility criteria, and all had a low risk of bias. A meta-analysis revealed a statistically significant increase in the mean CAC change for conjunctival itching, with low heterogeneity among measurements at 3 minutes (P < 0.00001, I2 = 47%), 5 minutes (P < 0.00001, I2 = 46%), and 7 minutes (P < 0.00001, I2 = 41%). Additionally, the meta-analysis found a statistically significant increase in the mean CAC change for conjunctival redness with low heterogeneity (P < 0.00001, I2 = 15%). The pooled analysis showed no significant difference (P = 0.57, I2 = 0%) between the DEXTENZA and placebo groups in the frequency of adverse events.
Conclusion: DEXTENZA has emerged as a promising and viable treatment option for patients with seasonal/perennial allergic conjunctivitis and is an effective alternative to current therapeutic modalities.
Keywords: DEXTENZA; allergic conjunctivitis; conjunctivitis; dexamethasone insert.
© 2024 Alsudais et al.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures


Similar articles
-
Vehicle-Controlled, Phase 2 Clinical Trial of a Sustained-Release Dexamethasone Intracanalicular Insert in a Chronic Allergen Challenge Model.J Ocul Pharmacol Ther. 2017 Mar;33(2):79-90. doi: 10.1089/jop.2016.0154. Epub 2017 Jan 10. J Ocul Pharmacol Ther. 2017. PMID: 28072552 Clinical Trial.
-
Phase 3 Randomized Study of Efficacy and Safety of a Dexamethasone Intracanalicular Insert in Patients With Allergic Conjunctivitis.Am J Ophthalmol. 2021 Sep;229:288-300. doi: 10.1016/j.ajo.2021.03.017. Epub 2021 Mar 25. Am J Ophthalmol. 2021. PMID: 33773984 Clinical Trial.
-
Effect of alcaftadine 0.25% on ocular itch associated with seasonal or perennial allergic conjunctivitis: a pooled analysis of two multicenter randomized clinical trials.Clin Ophthalmol. 2015 May 2;9:765-72. doi: 10.2147/OPTH.S80503. eCollection 2015. Clin Ophthalmol. 2015. PMID: 25999684 Free PMC article.
-
Second-generation antidepressants for treatment of seasonal affective disorder.Cochrane Database Syst Rev. 2021 Mar 4;3(3):CD008591. doi: 10.1002/14651858.CD008591.pub3. Cochrane Database Syst Rev. 2021. PMID: 33661528 Free PMC article.
-
Interventions for treatment of COVID-19: A living systematic review with meta-analyses and trial sequential analyses (The LIVING Project).PLoS Med. 2020 Sep 17;17(9):e1003293. doi: 10.1371/journal.pmed.1003293. eCollection 2020 Sep. PLoS Med. 2020. PMID: 32941437 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

