Rapid formation of N ε-(carboxymethyl)lysine (CML) from ribose depends on glyoxal production by oxidation
- PMID: 39323732
- PMCID: PMC11420854
- DOI: 10.1039/d4cb00183d
Rapid formation of N ε-(carboxymethyl)lysine (CML) from ribose depends on glyoxal production by oxidation
Abstract
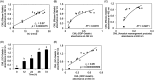
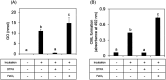
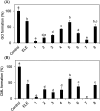
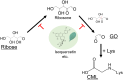
N ε-(Carboxymethyl)lysine (CML) is a major advanced glycation end-product (AGE) involved in protein dysfunction and inflammation in vivo. Its accumulation increases with age and is enhanced with the pathogenesis of diabetic complications. Therefore, the pathways involved in CML formation should be elucidated to understand the pathological conditions involved in CML. Ribose is widely used in glycation research because it shows a high reactivity with proteins to form AGEs. We previously demonstrated that ribose generates CML more rapidly than other reducing sugars, such as glucose; however, the underlying mechanism remains unclear. In this study, we focused on the pathway of CML formation from ribose. As a result, glyoxal (GO) was the most abundant product generated from ribose among the tested reducing sugars and was significantly correlated with CML formation from ribose-modified protein. The coefficient of determination (R 2) for CML formation between the ribose-modified protein and Amadori products or the ribose degradation product (RDP)-modified protein was higher for the RDP-modified protein. CML formation from ribose degradation products (RDP) incubated with protein significantly correlated with CML formation from GO-modified protein (r s = 0.95, p = 0.0000000869). GO and CML formation were inhibited by diethylenetriaminepentaacetic acid (DTPA) and enhanced by iron chloride. Additionally, flavonoid compounds such as isoquercetin, which are known to inhibit CML, also inhibited GO formation from ribose and CML formation. In conclusion, ribose undergoes auto-oxidation and oxidative cleavage between C-2 and C-3 to generate GO and enhance CML accumulation.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


Similar articles
-
Dietary glycation compounds - implications for human health.Crit Rev Toxicol. 2024 Sep;54(8):485-617. doi: 10.1080/10408444.2024.2362985. Epub 2024 Aug 16. Crit Rev Toxicol. 2024. PMID: 39150724
-
Free L-Lysine and Its Methyl Ester React with Glyoxal and Methylglyoxal in Phosphate Buffer (100 mM, pH 7.4) to Form Nε-Carboxymethyl-Lysine, Nε-Carboxyethyl-Lysine and Nε-Hydroxymethyl-Lysine.Int J Mol Sci. 2022 Mar 22;23(7):3446. doi: 10.3390/ijms23073446. Int J Mol Sci. 2022. PMID: 35408807 Free PMC article.
-
Peroxynitrite induces formation of N( epsilon )-(carboxymethyl) lysine by the cleavage of Amadori product and generation of glucosone and glyoxal from glucose: novel pathways for protein modification by peroxynitrite.Diabetes. 2002 Sep;51(9):2833-9. doi: 10.2337/diabetes.51.9.2833. Diabetes. 2002. PMID: 12196478
-
Protein Modification with Ribose Generates Nδ-(5-hydro-5-methyl-4-imidazolone-2-yl)-ornithine.Int J Mol Sci. 2022 Jan 22;23(3):1224. doi: 10.3390/ijms23031224. Int J Mol Sci. 2022. PMID: 35163152 Free PMC article.
-
Advanced glycation endproducts in food and their effects on health.Food Chem Toxicol. 2013 Oct;60:10-37. doi: 10.1016/j.fct.2013.06.052. Epub 2013 Jul 16. Food Chem Toxicol. 2013. PMID: 23867544 Review.
Cited by
-
Effects of C-Ring Structural Differences on the Inhibition of Nε-(Carboxyethyl)lysine in the Methylglyoxal-Lysine System by Flavonoids.Int J Mol Sci. 2025 Jun 19;26(12):5914. doi: 10.3390/ijms26125914. Int J Mol Sci. 2025. PMID: 40565377 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

