Development of a novel intramuscular liposomal injection for advanced meloxicam delivery: Preparation, characterization, in vivo pharmacokinetics, pharmacodynamics, and pain assessment in an orthopedic pain model
- PMID: 39323733
- PMCID: PMC11422154
- DOI: 10.1016/j.ijpx.2024.100284
Development of a novel intramuscular liposomal injection for advanced meloxicam delivery: Preparation, characterization, in vivo pharmacokinetics, pharmacodynamics, and pain assessment in an orthopedic pain model
Abstract
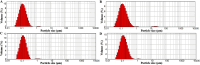
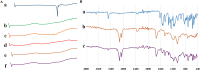
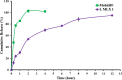
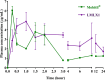
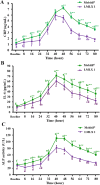
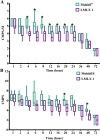
Pain produces several physiological, and degenerative complications. This study aimed to formulate meloxicam (MLX) in liposomes to increase solubility and deliver MLX in a controlled manner to overcome its poor aqueous solubility and relatively short t1/2 problems. Liposomes were prepared by thin film hydration followed by ultrasonication. Tests for characterizing formulations included particle size, span, entrapment efficiency, drug loading, stability, differential scanning calorimetry (DSC), Fourier transformation infrared (FT-IR) spectroscopy, morphology, in vitro release, release kinetics mathematical modeling, and an in vivo pain model in dogs undergoing orthopedic surgeries, followed by in vivo pharmacokinetics, pharmacodynamics, and pain assessment studies in comparison to the reference standard, Mobitil®. Liposomal MLX had a particle size of around 100 nm, 82 % entrapment efficiency, and 4.62 % drug loading. Stability studies, DSC, and FT-IR spectroscopy indicated that liposomes were highly stable. The formulation showed an improved in vitro controlled release pattern and an enhanced in vivo pharmacokinetic behavior as manifested by higher t1/2 and AUC0 - 24 and lower Cl/F in comparison to Mobitil®. The pharmacodynamics study and pain scales demonstrated liposomal MLX managed postoperative pain better than Mobitil®. In conclusion, the incorporation of MLX in liposomes increased its solubility and stability, as well as its pain management properties.
Keywords: CMPS-SF pain scale; Interleukin-6; Liposomes; Meloxicam; Pain management; Pharmacokinetics.
© 2024 The Authors. Published by Elsevier B.V.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



Similar articles
-
PEGylated Liposomes of Meloxicam: Optimization by Quality by Design, in vitro Characterization and Cytotoxicity Evaluation.Pharm Nanotechnol. 2017;5(2):119-137. doi: 10.2174/2211738505666170428152129. Pharm Nanotechnol. 2017. PMID: 28462699
-
Meloxicam-loaded Phospholipid/solutol® HS15 Based Mixed Nanomicelles: Preparation, Characterization, and in vitro Antioxidant Activity.Pharm Nanotechnol. 2016;4(3):167-190. doi: 10.2174/2211738504666160720162323. Pharm Nanotechnol. 2016. PMID: 29052497
-
Development of engineered transferosomal gel containing meloxicam for the treatment of osteoarthritis.Ann Pharm Fr. 2024 Sep;82(5):830-839. doi: 10.1016/j.pharma.2024.04.006. Epub 2024 Apr 22. Ann Pharm Fr. 2024. PMID: 38657858
-
Intra-articular injection PLGA blends sustained-release microspheres loaded with meloxicam: preparation, optimization, evaluation in vitro and in vivo.Drug Deliv. 2022 Dec;29(1):3317-3327. doi: 10.1080/10717544.2022.2144545. Drug Deliv. 2022. PMID: 36369759 Free PMC article.
-
Elastic liposomes bearing meloxicam-beta-cyclodextrin for transdermal delivery.Curr Drug Deliv. 2008 Jul;5(3):207-14. doi: 10.2174/156720108784911677. Curr Drug Deliv. 2008. PMID: 18673264
Cited by
-
Development of a selective COX-2 inhibitor: from synthesis to enhanced efficacy via nano-formulation.RSC Adv. 2024 Oct 17;14(45):32721-32732. doi: 10.1039/d4ra06295g. eCollection 2024 Oct 17. RSC Adv. 2024. PMID: 39429925 Free PMC article.
-
Spanlastic Nano-Vesicles: A Novel Approach to Improve the Dissolution, Bioavailability, and Pharmacokinetic Behavior of Famotidine.Pharmaceuticals (Basel). 2024 Nov 29;17(12):1614. doi: 10.3390/ph17121614. Pharmaceuticals (Basel). 2024. PMID: 39770456 Free PMC article.
References
-
- Alomrani A., Badran M., Harisa G.I., Alhariri M., Alshamsan A., Alkholief M. The use of chitosan-coated flexible liposomes as a remarkable carrier to enhance the antitumor efficacy of 5-fluorouracil against colorectal cancer. Saudi Pharm J. 2019;27:603–611. doi: 10.1016/j.jsps.2019.02.008. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

