Multifunctional bioactive glass nanoparticles: surface-interface decoration and biomedical applications
- PMID: 39323748
- PMCID: PMC11422188
- DOI: 10.1093/rb/rbae110
Multifunctional bioactive glass nanoparticles: surface-interface decoration and biomedical applications
Abstract
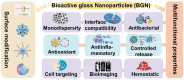
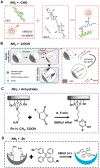
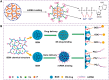
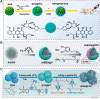
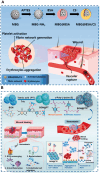
Developing bioactive materials with multifunctional properties is crucial for enhancing their biomedical applications in regenerative medicine. Bioactive glass nanoparticle (BGN) is a new generation of biomaterials that demonstrate high biocompatibility and tissue-inducing capacity. However, the hard nanoparticle surface and single surface property limited their wide biomedical applications. In recent years, the surface functional strategy has been employed to decorate the BGN and improve its biomedical applications in bone tissue repair, bioimaging, tumor therapy and wound repair. This review summarizes the progress of surface-interface design strategy, customized multifunctional properties and biomedical applications in detail. We also discussed the current challenges and further development of multifunctional BGN to meet the requirements of various biomedical applications.
Keywords: bioactive glass nanoparticles; functionalization; multifunctional properties; surface modification.
© The Author(s) 2024. Published by Oxford University Press.
Figures




Similar articles
-
Engineering multifunctional bioactive citrate-based biomaterials for tissue engineering.Bioact Mater. 2022 May 7;19:511-537. doi: 10.1016/j.bioactmat.2022.04.027. eCollection 2023 Jan. Bioact Mater. 2022. PMID: 35600971 Free PMC article. Review.
-
Sol-gel processing of bioactive glass nanoparticles: A review.Adv Colloid Interface Sci. 2017 Nov;249:363-373. doi: 10.1016/j.cis.2017.03.008. Epub 2017 Mar 21. Adv Colloid Interface Sci. 2017. PMID: 28364954 Review.
-
Monodisperse photoluminescent and highly biocompatible bioactive glass nanoparticles for controlled drug delivery and cell imaging.J Mater Chem B. 2015 May 14;3(18):3831-3839. doi: 10.1039/c5tb00204d. Epub 2015 Apr 13. J Mater Chem B. 2015. PMID: 32262857
-
Engineering a Biodegradable Multifunctional Antibacterial Bioactive Nanosystem for Enhancing Tumor Photothermo-Chemotherapy and Bone Regeneration.ACS Nano. 2020 Jan 28;14(1):442-453. doi: 10.1021/acsnano.9b06145. Epub 2019 Dec 24. ACS Nano. 2020. PMID: 31702885
-
Antioxidant cerium ions-containing mesoporous bioactive glass ultrasmall nanoparticles: Structural, physico-chemical, catalase-mimic and biological properties.Colloids Surf B Biointerfaces. 2021 Oct;206:111932. doi: 10.1016/j.colsurfb.2021.111932. Epub 2021 Jun 18. Colloids Surf B Biointerfaces. 2021. PMID: 34175740
References
-
- Hench LL, Polak JM.. Third-generation biomedical materials. Science 2002;295:1014–7. - PubMed
-
- Jones JR. Reprint of: review of bioactive glass: from hench to hybrids. Acta Biomater 2015;23 Suppl:S53–82. - PubMed
-
- Hench LL, Splinter RJ, Allen WC, Greenlee TK.. Bonding mechanisms at the interface of ceramic prosthetic materials. J Biomed Mater Res 1971;5:117–41.
-
- Blencke BA, Bromer H, Deutscher KK.. Compatibility and long-term stability of glass-ceramic implants. J Biomed Mater Res 1978;12:307–16. - PubMed
-
- Kokubo T, Ito S, Shigematsu M, Sakka S, Yamamuro T.. Mechanical-properties of a new type of apatite-containing glass ceramic for prosthetic application. J Mater Sci 1985;20:2001–4.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

