Utility of dynamic contrast enhancement for clinically significant prostate cancer detection
- PMID: 39323923
- PMCID: PMC11420102
- DOI: 10.1002/bco2.415
Utility of dynamic contrast enhancement for clinically significant prostate cancer detection
Erratum in
-
Erratum.BJUI Compass. 2024 Dec 30;5(12):1324-1329. doi: 10.1002/bco2.482. eCollection 2024 Dec. BJUI Compass. 2024. PMID: 39744071 Free PMC article.
Abstract
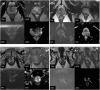
Objective: This study aimed to evaluate the association of dynamic contrast enhancement (DCE) with clinically significant prostate cancer (csPCa, Gleason Grade Group ≥2) and compare biparametric magnetic resonance imaging (bpMRI) and multiparametric MRI (mpMRI) nomograms.
Subjects/patients and methods: We identified a retrospective cohort of biopsy naïve patients who underwent pre-biopsy MRI separated by individual MRI series from 2018 to 2022. csPCa detection rates were calculated for patients with peripheral zone (PZ) lesions scored 3-5 on diffusion weighted imaging (DWI) with available DCE (annotated as - or +). bpMRI Prostate Imaging Reporting and Data System (PIRADS) (3 = 3-, 3+; 4 = 4-, 4+; 5 = 5-, 5+) and mpMRI PIRADS (3 = 3-; 4 = 3+, 4-, 4+; 5 = 5-, 5+) approaches were compared in multivariable logistic regression models. Nomograms for detection of csPCa and ≥GG3 PCa incorporating all biopsy naïve patients who underwent prostate MRI were generated based on available serum biomarkers [PHI, % free prostate-specific antigen (PSA), or total PSA] and validated with an independent cohort.
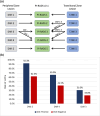
Results: Patients (n = 1010) with highest PIRADS lesion in PZ were included in initial analysis with 127 (12.6%) classified as PIRADS 3+ (PIRADS 3 on bpMRI but PIRADS 4 on mpMRI). On multivariable analysis, PIRADS 3+ lesions were associated with higher csPCa rates compared to PIRADS 3- (3+ vs. 3-: OR 1.86, p = 0.024), but lower csPCa rates compared to PIRADS DWI 4 lesions (4 vs. 3+: OR 2.39, p < 0.001). csPCa rates were 19% (3-), 31% (3+), 41.5% (4-), 65.9% (4+), 62.5% (5-), and 92.3% (5+). bpMRI nomograms were non-inferior to mpMRI nomograms in the development (n = 1410) and independent validation (n = 353) cohorts. Risk calculators available at: https://rossnm1.shinyapps.io/MynMRIskCalculator/.
Conclusion: While DCE positivity by itself was associated with csPCa among patients with highest PIRADS lesions in the PZ, nomogram comparisons suggest that there is no significant difference in performance of bpMRI and mpMRI. bpMRI may be considered as an alternative to mpMRI for prostate cancer evaluation in many situations.
Keywords: diagnosis; dynamic contrast enhancement; nomogram; prostate MRI; prostate cancer; risk stratification.
© 2024 The Author(s). BJUI Compass published by John Wiley & Sons Ltd on behalf of BJU International Company.
Conflict of interest statement
EMS is a consultant for Atria Academy of Science and Medicine, Early Medical, Lantheus, Pfizer, and PinnacleCare Health Advisors. AER is a consultant for Astellas, Astra Zeneca, Bayer HealthCare Pharmaceuticals, BillionToOne, Janssen Biotech, Lantheus, Myovant, Novartis, Pfizer, and Veracyte. These relationships are not related to the contents of this manuscript.
Figures
Similar articles
-
Optimizing detection of clinically significant prostate cancer through nomograms incorporating mri, clinical features, and advanced serum biomarkers in biopsy naïve men.Prostate Cancer Prostatic Dis. 2023 Sep;26(3):588-595. doi: 10.1038/s41391-023-00660-8. Epub 2023 Mar 27. Prostate Cancer Prostatic Dis. 2023. PMID: 36973367
-
Comparison of biparametric and multiparametric MRI in the diagnosis of prostate cancer.Cancer Imaging. 2019 Dec 21;19(1):90. doi: 10.1186/s40644-019-0274-9. Cancer Imaging. 2019. PMID: 31864408 Free PMC article.
-
Investigating the equivalent performance of biparametric compared to multiparametric MRI in detection of clinically significant prostate cancer.Abdom Radiol (NY). 2020 Feb;45(2):547-555. doi: 10.1007/s00261-019-02281-z. Abdom Radiol (NY). 2020. PMID: 31907568
-
Current Status of Biparametric MRI in Prostate Cancer Diagnosis: Literature Analysis.Life (Basel). 2022 May 28;12(6):804. doi: 10.3390/life12060804. Life (Basel). 2022. PMID: 35743835 Free PMC article. Review.
-
Biparametric vs multiparametric prostate magnetic resonance imaging for the detection of prostate cancer in treatment-naïve patients: a diagnostic test accuracy systematic review and meta-analysis.BJU Int. 2019 Aug;124(2):209-220. doi: 10.1111/bju.14759. Epub 2019 Apr 25. BJU Int. 2019. PMID: 30929292
Cited by
-
Erratum.BJUI Compass. 2024 Dec 30;5(12):1324-1329. doi: 10.1002/bco2.482. eCollection 2024 Dec. BJUI Compass. 2024. PMID: 39744071 Free PMC article.
References
-
- Brant A, Wu X, Prunty M, Margolis DJ, Bittencourt LK, Shoag JE, et al. Variability of prostate MRI charges among U.S. hospital‐based facilities. AJR Am J Roentgenol. 2023;1–2. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
