Performance of PSMA-targeted radiotheranostics in an experimental model of renal cell carcinoma
- PMID: 39324008
- PMCID: PMC11423292
- DOI: 10.3389/fonc.2024.1432286
Performance of PSMA-targeted radiotheranostics in an experimental model of renal cell carcinoma
Abstract
Introduction: Renal cell carcinoma (RCC) represents cancer originating from the renal epithelium and accounts for > 90% of cancers in the kidney. Prostate-specific membrane antigen (PSMA) is overexpressed in tumor-associated neovascular endothelial cells of many solid tumors, including metastatic RCC. Although studied in several small clinical studies, PSMA-based imaging and therapy have not been pursued rigorously in preclinical RCC. This study aimed to evaluate the preclinical performance of PSMA-based radiotheranostic agents in a relevant murine model.
Methods: A PSMA-overexpressing murine cell line, PSMA+ RENCA, was developed by lentiviral transduction. PSMA-based theranostic agents, 68Ga-L1/177Lu-L1/225Ac-L1, were synthesized in high radiochemical yield and purity following our reported methods. Immunocompetent BALB/c mice were used for flank and orthotopic tumor inoculation. 68Ga-L1 was evaluated in small animal PET/CT imaging in flank and PET/MR imaging in orthotopic models. Cell viability studies were conducted for 177Lu-L1 and 225Ac-L1. Proof-of-concept treatment studies were performed using 225Ac-L1 (0, 37 kBq, 2 kBq × 37 kBq, 1 week apart) using PSMA+ RENCA in the flank model.
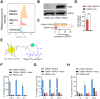
Results: Cellular uptake of 68Ga-L1, 177Lu-L1, and 225Ac-L1 confirmed the specificity of the agents to PSMA+ RENCA cells rather than to RENCA (wt) cells, which are low in PSMA expression. The uptake in PSMA+ RENCA cells at 1 h for 68Ga-L1 (49.0% incubated dose [ID] ± 3.6%ID/million cells), 177Lu-L1 (22.1%ID ± 0.5%ID)/million cells), and 225Ac-L1 (4.1% ± 0.2% ID)/million cells), respectively, were higher than the RENCA (wt) cells (~ 1%ID-2%ID/million cells). PET/CT images displayed > 7-fold higher accumulation of 68Ga-L1 in PSMA+ RENCA compared to RENCA (wt) in flank implantation at 1 h. A twofold higher accumulation of 68Ga-L1 was observed in orthotopic tumors than in normal kidneys during 1-3 h postinjection. High lung uptake was observed with 68Ga-L1 PET/MR imaging 3 weeks after orthotopic implantation of PSMA+ RENCA due to spontaneous lung metastases. The imaging data were further confirmed by immunohistochemical characterization. 225Ac-L1 (0-37 kBq) displayed a dose-dependent reduction of cell proliferation in the PSMA+ RENCA cells after 48 h incubation; ~ 40% reduction in the cells with treated 37 kBq compared to vehicle (p < 0.001); however, no effect was observed with 177Lu-L1 (0-3700 kBq) up to 144 h postinoculation, suggesting lower efficacy of β-particle-emitting radiations in cellular studies compared to α-particle-emitting 225Ac-L1. Animals treated with 225Ac-L1 at 1 week posttumor inoculation in flank models displayed significant tumor growth delay (p < 0.03) and longer median survival of 21 days and 24 days for the treatment groups 37 kBq and 2 kBq × 37 kBq, respectively, compared to the vehicle group (12 days).
Conclusion: The results suggest that a theranostic strategy targeting PSMA, employing PET and α-emitting radiopharmaceuticals, enabled tumor growth control and enhanced survival in a relevant immunocompetent murine model of RCC. These studies provide the rationale for clinical studies of PSMA-targeted theranostic agents in patients with RCC.
Keywords: actinium-225; alpha-particle emitting radionuclide; gallium-68; lutetium-177; positron-emission tomography; prostate-specific membrane antigen; targeted radiopharmaceutical therapy; β-particle emitting radionuclide.
Copyright © 2024 Singh, Thotakura, Alati, Lisok, Jiang, Merino, Minn, Yadav, Markowski, Ged, Pavlovich, Singla, Solnes, Gorin, Pomper, Rowe and Banerjee.
Conflict of interest statement
SRB, IM, and MP are coinventors on one or more US patents covering compounds discussed in this submission. They are entitled to a portion of any licensing fees and royalties generated by this technology. This arrangement has been reviewed and approved by Johns Hopkins University, following its conflict-of-interest policies. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Key Statistics About Kidney Cancer. New York City, USA: American Cancer Society; (2024). Available at: https://www.cancer.org/cancer/types/kidney-cancer/about/key-statistics.html.
-
- Chang SS, Bander NH, Heston WDW. Biology of Psma as a Diagnostic and Therapeutic Target. In: Klein EA, editor. Management of Prostate Cancer. Humana Press, Totowa, NJ: (2004). p. 609–30.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

