Inhibition of PCSK9: A Promising Enhancer for Anti-PD-1/PD-L1 Immunotherapy
- PMID: 39324018
- PMCID: PMC11423609
- DOI: 10.34133/research.0488
Inhibition of PCSK9: A Promising Enhancer for Anti-PD-1/PD-L1 Immunotherapy
Abstract
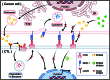
Immune checkpoint therapy, such as programmed cell death protein 1/programmed death-ligand 1 (PD-1/PD-L1) blockade, has achieved remarkable results in treating various tumors. However, most cancer patients show a low response rate to PD-1/PD-L1 blockade, especially those with microsatellite stable/mismatch repair-proficient colorectal cancer subtypes, which indicates an urgent need for new approaches to augment the efficacy of PD-1/PD-L1 blockade. Cholesterol metabolism, which involves generating multifunctional metabolites and essential membrane components, is also instrumental in tumor development. In recent years, inhibiting proprotein convertase subtilisin/kexin type 9 (PCSK9), a serine proteinase that regulates cholesterol metabolism, has been demonstrated to be a method enhancing the antitumor effect of PD-1/PD-L1 blockade to some extent. Mechanistically, PCSK9 inhibition can maintain the recycling of major histocompatibility protein class I, promote low-density lipoprotein receptor-mediated T-cell receptor recycling and signaling, and modulate the tumor microenvironment (TME) by affecting the infiltration and exclusion of immune cells. These mechanisms increase the quantity and enhance the antineoplastic effect of cytotoxic T lymphocyte, the main functional immune cells involved in anti-PD-1/PD-L1 immunotherapy, in the TME. Therefore, combining PCSK9 inhibition therapy with anti-PD-1/PD-L1 immunotherapy may provide a novel option for improving antitumor effects and may constitute a promising research direction. This review concentrates on the relationship between PCSK9 and cholesterol metabolism, systematically discusses how PCSK9 inhibition potentiates PD-1/PD-L1 blockade for cancer treatment, and highlights the research directions in this field.
Copyright © 2024 Shengbo Sun et al.
Conflict of interest statement
Competing interests: The authors declare that they have no competing interests.
Figures
Similar articles
-
A new enhancer for anti-PD-1/PD-L1 immunotherapy: PCSK9 inhibition.Trends Cancer. 2025 Feb;11(2):84-87. doi: 10.1016/j.trecan.2024.10.002. Epub 2024 Oct 24. Trends Cancer. 2025. PMID: 39455406 Review.
-
Methionine Metabolism Dictates PCSK9 Expression and Antitumor Potency of PD-1 Blockade in MSS Colorectal Cancer.Adv Sci (Weinh). 2025 May;12(19):e2501623. doi: 10.1002/advs.202501623. Epub 2025 Mar 24. Adv Sci (Weinh). 2025. PMID: 40125618 Free PMC article.
-
Inhibition of PCSK9 enhances the antitumor effect of PD-1 inhibitor in colorectal cancer by promoting the infiltration of CD8+ T cells and the exclusion of Treg cells.Front Immunol. 2022 Aug 8;13:947756. doi: 10.3389/fimmu.2022.947756. eCollection 2022. Front Immunol. 2022. PMID: 36003387 Free PMC article.
-
Functionalized biomimetic nanoparticles combining programmed death-1/programmed death-ligand 1 blockade with photothermal ablation for enhanced colorectal cancer immunotherapy.Acta Biomater. 2023 Feb;157:451-466. doi: 10.1016/j.actbio.2022.11.043. Epub 2022 Nov 25. Acta Biomater. 2023. PMID: 36442821
-
Application of PD-1 Blockade in Cancer Immunotherapy.Comput Struct Biotechnol J. 2019 May 23;17:661-674. doi: 10.1016/j.csbj.2019.03.006. eCollection 2019. Comput Struct Biotechnol J. 2019. PMID: 31205619 Free PMC article. Review.
Cited by
-
Monoclonal antibody immune therapy response instrument for stratification and cost-effective personalized approaches in 3PM-guided pan cancer management.EPMA J. 2025 Mar 22;16(2):465-503. doi: 10.1007/s13167-025-00403-w. eCollection 2025 Jun. EPMA J. 2025. PMID: 40438490 Review.
-
A literature review of recent advances in gastric cancer treatment: exploring the cross-talk between targeted therapies.Cancer Cell Int. 2025 Jan 24;25(1):23. doi: 10.1186/s12935-025-03655-8. Cancer Cell Int. 2025. PMID: 39856676 Free PMC article. Review.
-
Targeting proprotein convertase subtilisin/kexin type 9 (PCSK9) to tackle central nervous system diseases: role as a promising approach.Eur J Med Res. 2025 Jul 30;30(1):690. doi: 10.1186/s40001-025-02937-1. Eur J Med Res. 2025. PMID: 40739673 Free PMC article. Review.
-
Genetic Variants, Bioactive Compounds, and PCSK9 Inhibitors in Hyper-LDL-Cholesterolemia: A GWAS and In Silico Study on Cardiovascular Disease Risk.Nutrients. 2025 Apr 23;17(9):1411. doi: 10.3390/nu17091411. Nutrients. 2025. PMID: 40362720 Free PMC article.
-
A Critical Review of Immunomodulation in the Management of Inoperable Stage III NSCLC.Cancers (Basel). 2025 May 30;17(11):1829. doi: 10.3390/cancers17111829. Cancers (Basel). 2025. PMID: 40507315 Free PMC article. Review.
References
-
- Ramos CC, Pires J, Gonzalez E, Garcia-Vallicrosa C, Reis CA, Falcon-Perez JM, Freitas D. Extracellular vesicles in tumor-adipose tissue crosstalk: Key drivers and therapeutic targets in cancer cachexia. Extracell Vesicles Circ Nucl Acids. 2024;5:471–496.
-
- Yang Z, Zhang X, Zhang J, Gao J, Bai Z, Deng W, Chen G, An Y, Wei Q, Han J, et al. . Rationale and design of a prospective, multicenter, phase II clinical trial of safety and efficacy evaluation of long course neoadjuvant chemoradiotherapy plus tislelizumab followed by total mesorectal excision for locally advanced rectal cancer (NCRT-PD1-LARC trial). BMC Cancer. 2022;22(1):462. - PMC - PubMed
-
- Yang Z, Ma J, Han J, Li A, Liu G, Sun Y, Zheng J, Zhang J, Chen G, Xu R, et al. . Gut microbiome model predicts response to neoadjuvant immunotherapy plus chemoradiotherapy in rectal cancer. Med. 2023;5:1–14. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

