Mechanism and toxicity assessment of carbofuran degradation by persulfate-based advanced oxidation process
- PMID: 39324045
- PMCID: PMC11421621
- DOI: 10.1039/d4ra05365f
Mechanism and toxicity assessment of carbofuran degradation by persulfate-based advanced oxidation process
Abstract
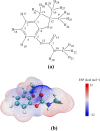
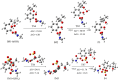
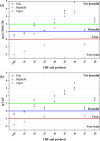
The advanced oxidation process based on persulfate has been proven to be a promising method for degrading the highly toxic carbamate pesticide carbofuran (CBF). However, the mechanism of CBF degradation by sulfate radicals (SO4·-) and hydroxyl radicals (·OH) is still unclear and requires further research and discussion. This study investigated the mechanism and toxicity assessment of CBF degradation using density functional theory (DFT) theory calculation methods. The results indicated that SO4·- and ·OH can undergo addition and abstraction reactions with CBF. Thermodynamic and kinetic analysis showed that the abstraction reaction between SO4·- and the secondary H atom is the optimal reaction pathway, exhibiting the highest branching ratio (Γ = 41.84%). The rate constants for the reactions of CBF with SO4·- and ·OH at room temperature were found to be 3.66 × 109 and 8.96 × 108 M-1 s-1, respectively, which are consistent with experimental data reported in previous studies. The acute and chronic toxicity of CBF and its degradation products to aquatic organisms was predicted through an ecological toxicity assessment model. The toxicity of the degradation products was lower than that of the parent CBF, confirming the viability of using persulfate-based advanced oxidation processes for water treatment.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures






References
-
- Lan J. Sun W. Chen L. Zhou H. Fan Y. Diao X. Wang B. Zhao H. Food Agric. Immunol. 2020;31:165–175.
-
- Gupta J. Rathour R. Singh R. Thakur I. S. Bioresour. Technol. 2019;282:417–424. - PubMed
-
- Abad A. Moreno M. J. Montoya A. J. Agric. Food Chem. 1999;47:2475–2485. - PubMed
-
- Otieno P. O. Lalah J. O. Virani M. Jondiko I. O. Schramm K. W. J. Environ. Sci. Health, Part B. 2010;45:137–144. - PubMed
LinkOut - more resources
Full Text Sources

