Effects of interleukin-1 receptor antagonism in women with polycystic ovary syndrome-the FertIL trial
- PMID: 39324125
- PMCID: PMC11423739
- DOI: 10.3389/fendo.2024.1435698
Effects of interleukin-1 receptor antagonism in women with polycystic ovary syndrome-the FertIL trial
Abstract
Introduction: Chronic low-grade inflammation might contribute to hyperandrogenemia and metabolic complications in polycystic ovary syndrome (PCOS). The proinflammatory cytokine interleukin (IL)-1 stimulates androgen production from ovarian cells, whereas blockade of the IL-1 pathway improves cardiometabolic health. We aimed to investigate whether blocking the IL-1 pathway ameliorates hyperandrogenemia in patients with PCOS.
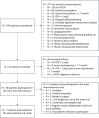
Methods: This is a prospective, interventional, single-arm, proof-of-concept trial performed at a tertiary hospital in Switzerland (August 2018 to July 2020) in 18 premenopausal women with a diagnosis of PCOS according to the Rotterdam criteria, total testosterone levels ≥ 1.7 nmol/L, and C-reactive protein (CRP) ≥ 1.0 mg/L. Patients received 100 mg/day of the IL-1-receptor antagonist anakinra for 28 days and underwent weekly blood sampling until 1 week after the end of treatment. The primary endpoint was the change in serum androstenedione levels on day 7 of treatment, assessed with liquid chromatography-tandem mass spectrometry. Seven of these women participated in a subsequent observational sub-study (May 2021 to December 2021).
Results: Median [interquartile range (IQR)] androstenedione increased by 0.5 [-0.1, 1.6] nmol/L (p = 0.048) with anakinra and by 1.3 [0.08, 2.4] nmol/L [p = 0.38] without anakinra between baseline and day 7. Anakinra reduced CRP levels on days 7, 21, and 28 (p < 0.001) but did not lead to an absolute reduction in androgens. However, four of six patients (67%) had smaller areas under the curves for androstenedione and/or testosterone during the 28-day intervention with anakinra as compared to 28 days without treatment.
Discussion: Our findings suggest that anakinra suppresses IL-1-mediated chronic low-grade inflammation in PCOS and might attenuate biochemical hyperandrogenemia.
Keywords: PCOS; anakinra; hyperandrogenemia; inflammation; interleukin-1; polycystic ovary syndrome.
Copyright © 2024 Wälchli-Popovic, Monnerat, Taylor, Gilligan, Schiffer, Arlt, Vogt, De Geyter, Hutter, Donath, Sartorius and Christ-Crain.
Conflict of interest statement
Author GS was employed by company Fertisuisse. MD is listed as one of the inventors on a patent filed in 2003 for the use of IL-1 inhibiting drugs against type 2 diabetes. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Wild RA, Carmina E, Diamanti-Kandarakis E, Dokras A, Escobar-Morreale HF, Futterweit W, et al. . Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome (AE-PCOS) Society. J Clin Endocrinol Metab. (2010) 95:2038–49. doi: 10.1210/jc.2009-2724 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

