Role of cellular effectors in the induction and maintenance of IgA responses leading to protective immunity against enteric bacterial pathogens
- PMID: 39324143
- PMCID: PMC11422102
- DOI: 10.3389/fimmu.2024.1446072
Role of cellular effectors in the induction and maintenance of IgA responses leading to protective immunity against enteric bacterial pathogens
Abstract
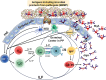
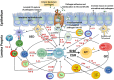
The mucosal immune system is a critical first line of defense to infectious diseases, as many pathogens enter the body through mucosal surfaces, disrupting the balanced interactions between mucosal cells, secretory molecules, and microbiota in this challenging microenvironment. The mucosal immune system comprises of a complex and integrated network that includes the gut-associated lymphoid tissues (GALT). One of its primary responses to microbes is the secretion of IgA, whose role in the mucosa is vital for preventing pathogen colonization, invasion and spread. The mechanisms involved in these key responses include neutralization of pathogens, immune exclusion, immune modulation, and cross-protection. The generation and maintenance of high affinity IgA responses require a delicate balance of multiple components, including B and T cell interactions, innate cells, the cytokine milieu (e.g., IL-21, IL-10, TGF-β), and other factors essential for intestinal homeostasis, including the gut microbiota. In this review, we will discuss the main cellular components (e.g., T cells, innate lymphoid cells, dendritic cells) in the gut microenvironment as mediators of important effector responses and as critical players in supporting B cells in eliciting and maintaining IgA production, particularly in the context of enteric infections and vaccination in humans. Understanding the mechanisms of humoral and cellular components in protection could guide and accelerate the development of more effective mucosal vaccines and therapeutic interventions to efficiently combat mucosal infections.
Keywords: IgA; T cells; enteric diseases; long term immune response; vaccination and controlled human infection model (CHIM).
Copyright © 2024 Carreto-Binaghi, Sztein and Booth.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Pakkanen SH, Kantele JM, Moldoveanu Z, Hedges S, Hakkinen M, Mestecky J, et al. . Expression of homing receptors on IgA1 and IgA2 plasmablasts in blood reflects differential distribution of IgA1 and IgA2 in various body fluids. Clin Vaccine immunol: CVI. (2010) 17:393–401. doi: 10.1128/CVI.00475-09 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

