A Nanosized Porous Supramolecular Lead(II)- N'-phenyl(pyridin-2-yl)methylene- N-phenylthiosemicarbazide Aggregate, Obtained Under Electrochemical Conditions
- PMID: 39324362
- PMCID: PMC11462507
- DOI: 10.1021/acs.inorgchem.4c02182
A Nanosized Porous Supramolecular Lead(II)- N'-phenyl(pyridin-2-yl)methylene- N-phenylthiosemicarbazide Aggregate, Obtained Under Electrochemical Conditions
Abstract
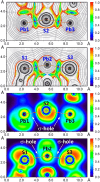
A novel nanosized porous supramolecular nonanuclear complex [Pb9(HL)12Cl2(ClO4)](ClO4)3·15H2O·a(solvent) (1·15H2O·a(solvent)) is reported that was synthesized by electrochemical oxidation of a Pb anode under the ambient conditions in a CH3CN:MeOH solution of N'-phenyl(pyridin-2-yl)methylene-N-phenylthiosemicarbazide (H2L), containing [N(CH3)4]ClO4 as a current carrier. The supramolecular aggregate of 1 is enforced by a myriad of Pb···S tetrel bonds (TtBs) established with the thiocarbonyl sulfur atoms of adjacent species, which have been also analyzed by DFT calculations via 2D maps of ELF, Laplacian and RDG properties. Moreover, Pb···Cl TtBs with the central Cl- anion, and Pb···O TtBs with the three oxygen atoms of the ClO4- anion, were revealed. Notably, the molecular structure of 1 differs significantly from that recently reported by us [Pb2(HL)2(CH3CN)(ClO4)2]·2H2O (2·2H2O), which was obtained using a conventional synthetic procedure by reacting Pb(ClO4)2 with H2L in the same CH3CN:MeOH solution, thus highlighting a crucial role of the electrochemical conditions. The optical characteristics of the complex were investigated using UV-vis spectroscopy and spectrofluorimetry in methanol. The complex was found to be emissive when excited at 304 nm, producing a broad emission band ranging from approximately 420 to 600 nm with multiple peaks. The CIE-1931 chromaticity coordinates, calculated as (0.33, 0.24), suggest that the emission lies in the white region of the chromaticity diagram. Further investigation is needed to fully characterize the origin of this emission.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Garnovskii A. D.; Kharisov B. I.; Gojon-Zorrilla G.; Garnovskii D. A. Direct synthesis of coordination compounds from zerovalent metals and organic ligands. Russ. Chem. Rev. 1995, 64, 201–221. 10.1070/RC1995v064n03ABEH000145. - DOI
-
- García-Vásquez J. A.; Romero J.; Sousa A. Electrochemical synthesis of metallic complexes of bidentate thiolates containing nitrogen as an additional donor atom. Coord. Chem. Rev. 1999, 193–195, 691–745. 10.1016/S0010-8545(99)00046-6. - DOI
-
- Garnovskii A. D.; Blanco L. M.; Kharisov B. I.; Garnovskii D. A.; Burlov A. S. Direct electrosynthesis of metal complexes: State of the art. J. Coord. Chem. 1999, 48, 219–263. 10.1080/00958979908024555. - DOI
-
- Kharisov B. I.; Garnovskii A. D.; Kharissova O. V.; Méndez U. O.; Tsivadze A. Y. Direct electrochemical synthesis of metal complexes of phthalocyanines and azomethines as model compounds: advantages and problems of this method versus traditional synthetic techniques. J. Coord. Chem. 2007, 60, 1435–1455. 10.1080/00958970601040658. - DOI
-
- Panyushkin V. T.; Kolokolov F. A.; Oflidi A. I.; Nazarenko M. A.. Electrochemical Synthesis of Coordination Compounds of Lanthanides: Effective Luminophores, In Handbook of Ecomaterials, Martínez L.; Kharissova O.; Kharisov B., Eds.; Springer: Cham, 2018.
LinkOut - more resources
Full Text Sources

