How type-2 dendritic cells induce Th2 differentiation: Instruction, repression, or fostering T cell-T cell communication?
- PMID: 39324367
- PMCID: PMC11804308
- DOI: 10.1111/all.16337
How type-2 dendritic cells induce Th2 differentiation: Instruction, repression, or fostering T cell-T cell communication?
Abstract
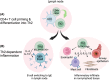
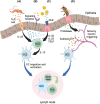
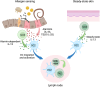
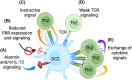
Allergic disease is caused by the activation of allergen-specific CD4+ type-2 T follicular helper cells (Tfh2) and T helper 2 (Th2) effector cells that secrete the cytokines IL-4, IL-5, IL-9, and IL-13 upon allergen encounter, thereby inducing IgE production by B cells and tissue inflammation. While it is accepted that the priming and differentiation of naïve CD4+ T cells into Th2 requires allergen presentation by type 2 dendritic cells (DC2s), the underlying signals remain unidentified. In this review we focus on the interaction between allergen-presenting DC2s and naïve CD4+ T cells in lymph node (LN), and the potential mechanisms by which DC2s might instruct Th2 differentiation. We outline recent advances in characterizing DC2 development and heterogeneity. We review mechanisms of allergen sensing and current proposed mechanisms of Th2 differentiation, with specific consideration of the role of DC2s and how they might contribute to each mechanism. Finally, we assess recent publications reporting a detailed analysis of DC-T cell interactions in LNs and how they support Th2 differentiation. Together, these studies are starting to shape our understanding of this key initial step of the allergic immune response.
Keywords: ILC2; Th2 differentiation; alarmins; allergen; dendritic cells.
© 2024 The Author(s). Allergy published by European Academy of Allergy and Clinical Immunology and John Wiley & Sons Ltd.
Conflict of interest statement
FR, GW, SO, OL and MB declare no conflict of interest.
Figures

References
-
- Jutel M, Agache I, Zemelka‐Wiacek M, et al. Nomenclature of allergic diseases and hypersensitivity reactions: adapted to modern needs: an EAACI position paper. Allergy. 2023;78(11):2851‐2874. - PubMed
-
- Rothenberg‐Lausell C, Bar J, Dahabreh D, Renert‐Yuval Y, Del Duca E, Guttman‐Yassky E. Biologic and small molecule therapy for treating moderate to severe atopic dermatitis: mechanistic considerations. J Allergy Clin Immunol. 2024;154:20‐30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

