Rodent models of tumours of the central nervous system
- PMID: 39324445
- PMCID: PMC11619804
- DOI: 10.1002/1878-0261.13729
Rodent models of tumours of the central nervous system
Abstract
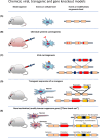
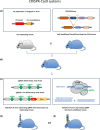
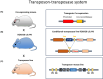
Modelling of human diseases is an essential component of biomedical research, to understand their pathogenesis and ultimately, develop therapeutic approaches. Here, we will describe models of tumours of the central nervous system, with focus on intrinsic CNS tumours. Model systems for brain tumours were established as early as the 1920s, using chemical carcinogenesis, and a systematic analysis of different carcinogens, with a more refined histological analysis followed in the 1950s and 1960s. Alternative approaches at the time used retroviral carcinogenesis, allowing a more topical, organ-centred delivery. Most of the neoplasms arising from this approach were high-grade gliomas. Whilst these experimental approaches did not directly demonstrate a cell of origin, the localisation and growth pattern of the tumours already pointed to an origin in the neurogenic zones of the brain. In the 1980s, expression of oncogenes in transgenic models allowed a more targeted approach by expressing the transgene under tissue-specific promoters, whilst the constitutive inactivation of tumour suppressor genes ('knock out')-often resulted in embryonic lethality. This limitation was elegantly solved by engineering the Cre-lox system, allowing for a promoter-specific, and often also time-controlled gene inactivation. More recently, the use of the CRISPR Cas9 technology has significantly increased experimental flexibility of gene expression or gene inactivation and thus added increased value of rodent models for the study of pathogenesis and establishing preclinical models.
Keywords: CRISPR Cas9 system; Cre‐lox system; brain tumour; genetically modified mouse model; glioblastoma; medulloblastoma.
© 2024 The Author(s). Molecular Oncology published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Cancer stem cells in the mammalian central nervous system.Cell Prolif. 2005 Dec;38(6):423-33. doi: 10.1111/j.1365-2184.2005.00358.x. Cell Prolif. 2005. PMID: 16300654 Free PMC article. Review.
-
Somatic CRISPR/Cas9-mediated tumour suppressor disruption enables versatile brain tumour modelling.Nat Commun. 2015 Jun 11;6:7391. doi: 10.1038/ncomms8391. Nat Commun. 2015. PMID: 26067104 Free PMC article.
-
An investigation of WNT pathway activation and association with survival in central nervous system primitive neuroectodermal tumours (CNS PNET).Br J Cancer. 2009 Apr 21;100(8):1292-302. doi: 10.1038/sj.bjc.6604979. Epub 2009 Mar 17. Br J Cancer. 2009. PMID: 19293793 Free PMC article.
-
CRISPR/Cas9 Technology as a Modern Genetic Manipulation Tool for Recapitulating of Neurodegenerative Disorders in Large Animal Models.Curr Gene Ther. 2021;21(2):130-148. doi: 10.2174/1566523220666201214115024. Curr Gene Ther. 2021. PMID: 33319680 Review.
-
Sonic hedgehog signalling pathway in CNS tumours: its role and therapeutic implications.Mol Brain. 2024 Nov 20;17(1):83. doi: 10.1186/s13041-024-01155-w. Mol Brain. 2024. PMID: 39568072 Free PMC article. Review.
Cited by
-
Understanding brain cancer and exploiting its vulnerabilities.Mol Oncol. 2024 Dec;18(12):2817-2821. doi: 10.1002/1878-0261.13769. Epub 2024 Nov 25. Mol Oncol. 2024. PMID: 39582358 Free PMC article.
References
-
- Seligman AM, Shear MJ, Alexander L. Studies in carcinogenesis: VIII. Experimental production of brain tumors in mice with Methylcholanthrene. Am J Cancer. 1939;37:364. 10.1158/ajc.1939.364 - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

