Real-world outcomes for high-risk non-muscle-invasive bladder cancer: screened patients for the BRAVO trial
- PMID: 39324506
- PMCID: PMC11745995
- DOI: 10.1111/bju.16516
Real-world outcomes for high-risk non-muscle-invasive bladder cancer: screened patients for the BRAVO trial
Abstract
Objective: To report real-world outcomes for high-risk non-muscle-invasive bladder cancer (HRNMIBC), including bacillus Calmette-Guérin (BCG) and radical cystectomy (RC), as randomised comparisons of these have not been possible.
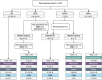
Methods: We detail consecutive participants screened for the BRAVO randomised controlled trial comparing RC with BCG (International Standard Randomised Controlled Trial Number [ISRCTN]12509361). Patients were prospectively registered and case-note review used for outcomes. The primary outcome was overall survival. Secondary outcomes included recurrence, progression, metastasis, and bladder cancer-specific survival.
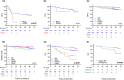
Results and limitations: A total of 193 patients were screened, including 106 (54.9%) who received BCG, 43 (22.3%) primary RC, 37 (19.2%) 'other' treatment and seven (3.6%) hyperthermic intravesical mitomycin C. All-cause death occurred in 55 (28.5%) patients at median (interquartile range [IQR]) of 29.0 (19.5-42.0) months. In multivariable analysis, overall mortality was more common in older patients (hazard ratio [HR] 2.63, 95% confidence interval [CI] 1.35-5.13; Cox P = 0.004 for age >70 years), those recruited from district hospitals (HR 0.53, 95% CI 0.3-0.95; P = 0.032) and those who did not undergo RC as their first treatment (HR 2.16, 95% CI 1.17-3.99; P = 0.014). In all, 17 (8.8%) patients died from bladder cancer (BC) at median (IQR) of 22.5 (19-36.25) months. In multivariable analysis, BC-specific mortality was more common in older patients (HR 4.87, 95% CI 1.1-21.6; P = 0.037) and those with Tis/T1 disease (HR 2.26, 95% CI 1.23-4.16; P = 0.008) but did not vary with initial treatment.
Conclusions: Patients with HRNMIBC are at high-risk of mortality. Those choosing RC as their initial treatment have lower risks of mortality than others, although this may reflect fitness and selection.
Keywords: BCG; High‐risk non‐muscle‐invasive bladder cancer; RCT; bladder cancer; feasibility study; radical cystectomy; real‐world data; surgical trial.
© 2024 The Author(s). BJU International published by John Wiley & Sons Ltd on behalf of BJU International.
Figures
References
-
- Jubber I, Ong S, Bukavina L et al. Epidemiology of bladder cancer in 2023: a systematic review of risk factors. Eur Urol 2023; 84: 176–190 - PubMed
-
- Sylvester RJ, van der Meijden APM, Oosterlinck W et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol 2006; 49: 466–477 - PubMed
-
- Babjuk M, Burger M, Capoun O et al. European Association of Urology guidelines on non‐muscle‐invasive bladder cancer (Ta, T1, and carcinoma in situ). Eur Urol 2022; 81: 75–94 - PubMed
-
- Malmström P‐U, Sylvester RJ, Crawford DE et al. An individual patient data meta‐analysis of the long‐term outcome of randomised studies comparing intravesical mitomycin C versus bacillus Calmette‐Guérin for non–muscle‐invasive bladder cancer. Eur Urol 2009; 56: 247–256 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

