Muscle-resident mesenchymal progenitors sense and repair peripheral nerve injury via the GDNF-BDNF axis
- PMID: 39324575
- PMCID: PMC11426970
- DOI: 10.7554/eLife.97662
Muscle-resident mesenchymal progenitors sense and repair peripheral nerve injury via the GDNF-BDNF axis
Abstract
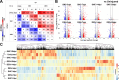
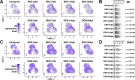
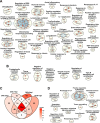
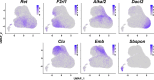
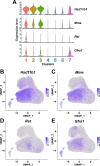
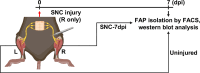
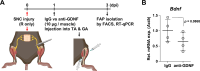
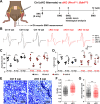
Fibro-adipogenic progenitors (FAPs) are muscle-resident mesenchymal progenitors that can contribute to muscle tissue homeostasis and regeneration, as well as postnatal maturation and lifelong maintenance of the neuromuscular system. Recently, traumatic injury to the peripheral nerve was shown to activate FAPs, suggesting that FAPs can respond to nerve injury. However, questions of how FAPs can sense the anatomically distant peripheral nerve injury and whether FAPs can directly contribute to nerve regeneration remained unanswered. Here, utilizing single-cell transcriptomics and mouse models, we discovered that a subset of FAPs expressing GDNF receptors Ret and Gfra1 can respond to peripheral nerve injury by sensing GDNF secreted by Schwann cells. Upon GDNF sensing, this subset becomes activated and expresses Bdnf. FAP-specific inactivation of Bdnf (Prrx1Cre; Bdnffl/fl) resulted in delayed nerve regeneration owing to defective remyelination, indicating that GDNF-sensing FAPs play an important role in the remyelination process during peripheral nerve regeneration. In aged mice, significantly reduced Bdnf expression in FAPs was observed upon nerve injury, suggesting the clinical relevance of FAP-derived BDNF in the age-related delays in nerve regeneration. Collectively, our study revealed the previously unidentified role of FAPs in peripheral nerve regeneration, and the molecular mechanism behind FAPs' response to peripheral nerve injury.
Keywords: BDNF; GDNF; fibro-adipogenic progenitor; mouse; neuroscience; peripheral nerve injury; regenerative medicine; schwann cell myelination; single-cell RNA-sequencing; stem cells.
© 2024, Yoo et al.
Conflict of interest statement
KY, YJ, TY, SH, IP, YK, YK, JR, IS, JK, DB, YK No competing interests declared
Figures



















Update of
- doi: 10.1101/2024.03.25.586563
- doi: 10.7554/eLife.97662.1
- doi: 10.7554/eLife.97662.2
Similar articles
-
GFRα1 Promotes Axon Regeneration after Peripheral Nerve Injury by Functioning as a Ligand.Adv Sci (Weinh). 2025 Jan;12(4):e2400812. doi: 10.1002/advs.202400812. Epub 2024 Dec 4. Adv Sci (Weinh). 2025. PMID: 39630029 Free PMC article.
-
Down-regulation of microRNA-216a confers protection against yttrium aluminium garnet laser-induced retinal injury via the GDNF-mediated GDNF/GFRα1/RET signalling pathway.J Biosci. 2018 Dec;43(5):985-1000. J Biosci. 2018. PMID: 30541958
-
Utilization of the Rat Tibial Nerve Transection Model to Evaluate Cellular and Molecular Mechanisms Underpinning Denervation-Mediated Muscle Injury.Int J Mol Sci. 2024 Feb 3;25(3):1847. doi: 10.3390/ijms25031847. Int J Mol Sci. 2024. PMID: 38339124 Free PMC article.
-
Schwann cells, neurotrophic factors, and peripheral nerve regeneration.Microsurgery. 1998;18(7):397-405. doi: 10.1002/(sici)1098-2752(1998)18:7<397::aid-micr2>3.0.co;2-f. Microsurgery. 1998. PMID: 9880154 Review.
-
Fibro-adipogenic progenitors in skeletal muscle homeostasis, regeneration and diseases.Open Biol. 2021 Dec;11(12):210110. doi: 10.1098/rsob.210110. Epub 2021 Dec 8. Open Biol. 2021. PMID: 34875199 Free PMC article. Review.
Cited by
-
Network pharmacology approach to unravel the neuroprotective potential of natural products: a narrative review.Mol Divers. 2025 Apr 25. doi: 10.1007/s11030-025-11198-3. Online ahead of print. Mol Divers. 2025. PMID: 40279084 Review.
-
Muscle fibroblasts and stem cells stimulate motor neurons in an age and exercise-dependent manner.Aging Cell. 2025 Mar;24(3):e14413. doi: 10.1111/acel.14413. Epub 2024 Nov 18. Aging Cell. 2025. PMID: 39555723 Free PMC article.
References
-
- Arthur-Farraj PJ, Latouche M, Wilton DK, Quintes S, Chabrol E, Banerjee A, Woodhoo A, Jenkins B, Rahman M, Turmaine M, Wicher GK, Mitter R, Greensmith L, Behrens A, Raivich G, Mirsky R, Jessen KR. c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron. 2012;75:633–647. doi: 10.1016/j.neuron.2012.06.021. - DOI - PMC - PubMed
-
- Bakooshli MA, Wang YX, Monti E, Su S, Kraft P, Nalbandian M, Alexandrova L, Wheeler JR, Vogel H, Blau HM. Regeneration of neuromuscular synapses after acute and chronic denervation by inhibiting the gerozyme 15-prostaglandin dehydrogenase. Science Translational Medicine. 2023;15:eadg1485. doi: 10.1126/scitranslmed.adg1485. - DOI - PMC - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

