Phase separation and viral factories: unveiling the physical processes supporting RNA packaging in dsRNA viruses
- PMID: 39324618
- PMCID: PMC11555692
- DOI: 10.1042/BST20231304
Phase separation and viral factories: unveiling the physical processes supporting RNA packaging in dsRNA viruses
Abstract
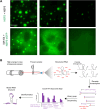
Understanding of the physicochemical properties and functions of biomolecular condensates has rapidly advanced over the past decade. More recently, many RNA viruses have been shown to form cytoplasmic replication factories, or viroplasms, via phase separation of their components, akin to numerous cellular membraneless organelles. Notably, diverse viruses from the Reoviridae family containing 10-12 segmented double-stranded RNA genomes induce the formation of viroplasms in infected cells. Little is known about the inner workings of these membraneless cytoplasmic inclusions and how they may support stoichiometric RNA assembly in viruses with segmented RNA genomes, raising questions about the roles of phase separation in coordinating viral genome packaging. Here, we discuss how the molecular composition of viroplasms determines their properties, highlighting the interplay between RNA structure, RNA remodelling, and condensate self-organisation. Advancements in RNA structural probing and theoretical modelling of condensates can reveal the mechanisms through which these ribonucleoprotein complexes support the selective enrichment and stoichiometric assembly of distinct viral RNAs.
Keywords: RNA structure; RNA:RNA interactions; RNP granules; X-ray RNA footprinting; viral factories.
© 2024 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures


Similar articles
-
Principles of RNA recruitment to viral ribonucleoprotein condensates in a segmented dsRNA virus.Elife. 2023 Jan 26;12:e68670. doi: 10.7554/eLife.68670. Elife. 2023. PMID: 36700549 Free PMC article.
-
Genome packaging in multi-segmented dsRNA viruses: distinct mechanisms with similar outcomes.Curr Opin Virol. 2018 Dec;33:106-112. doi: 10.1016/j.coviro.2018.08.001. Epub 2018 Aug 23. Curr Opin Virol. 2018. PMID: 30145433 Free PMC article. Review.
-
Seeing Biomolecular Condensates Through the Lens of Viruses.Annu Rev Virol. 2023 Sep 29;10(1):163-182. doi: 10.1146/annurev-virology-111821-103226. Epub 2023 Apr 11. Annu Rev Virol. 2023. PMID: 37040799 Review.
-
Liquid-liquid phase separation is essential for reovirus viroplasm formation and immune evasion.J Virol. 2024 Sep 17;98(9):e0102824. doi: 10.1128/jvi.01028-24. Epub 2024 Aug 28. J Virol. 2024. PMID: 39194247 Free PMC article.
-
Liquid-liquid Phase Separation in Viral Function.J Mol Biol. 2023 Aug 15;435(16):167955. doi: 10.1016/j.jmb.2023.167955. Epub 2023 Jan 13. J Mol Biol. 2023. PMID: 36642156 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

