Intermittent preventive treatment regimens for malaria in HIV-positive pregnant women
- PMID: 39324693
- PMCID: PMC11426187
- DOI: 10.1002/14651858.CD006689.pub3
Intermittent preventive treatment regimens for malaria in HIV-positive pregnant women
Abstract
Background: Malaria and HIV infection overlap geographically in sub-Saharan Africa and share risk factors. HIV infection increases malaria's severity, especially in pregnant women. The World Health Organization (WHO) recommends intermittent preventive treatment in pregnancy (IPTp) with sulphadoxine-pyrimethamine (SP) for pregnant women living in areas of stable malaria transmission. However, HIV-positive women on daily cotrimoxazole prophylaxis (recommended for prevention of opportunistic infections in people with HIV) cannot receive SP due to adverse drug interactions, so malaria prevention in this vulnerable population currently relies on daily cotrimoxazole prophylaxis alone. This review is based on a new protocol and provides an update to the 2011 Cochrane Review that evaluated alternative drugs for IPTp to prevent malaria in HIV-positive women.
Objectives: To compare the safety and efficacy of intermittent preventive treatment regimens for malaria prevention in HIV-positive pregnant women.
Search methods: We searched CENTRAL, MEDLINE, Embase, three other databases, and two trial registries to 31 January 2024. To identify relevant additional studies or unpublished work, we checked references and contacted study authors and other researchers working on malaria and HIV.
Selection criteria: We included randomized controlled trials (RCTs) comparing any intermittent preventive treatment regimen for preventing malaria in HIV-positive pregnant women against daily cotrimoxazole prophylaxis alone, placebo, current or previous standard of care, or combinations of these options. By 'standard of care' we refer to the country's recommended drug regimen to prevent malaria in pregnancy among HIV-positive women, or the treatment that a trial's research team considered to be the standard of care.
Data collection and analysis: Review authors, in pairs, independently screened all records identified by the search strategy, applied inclusion criteria, assessed risk of bias in included trials, and extracted data. We contacted trial authors when additional information was required. We presented dichotomous outcomes using risk ratios (RRs), count outcomes as incidence rate ratios (IRRs), and continuous outcomes as mean differences (MDs). We presented all measures of effect with 95% confidence intervals (CIs). We assessed the certainty of the evidence using the GRADE approach for what we considered to be the main comparisons and outcomes.
Main results: We included 14 RCTs, with a total of 4976 HIV-positive pregnant women initially randomized. All trials assessed the efficacy and safety of one antimalarial used as IPTp (mefloquine, dihydroartemisinin/piperaquine, SP, or azithromycin) with or without daily cotrimoxazole, compared to daily cotrimoxazole alone, placebo, or a standard of care regimen. We grouped the trials into nine comparisons. Our main comparison evaluated the current standard of care (daily cotrimoxazole) with another drug regimen (mefloquine or dihydroartemisinin/piperaquine) versus daily cotrimoxazole with or without placebo. In this comparison, two trials evaluated mefloquine and three evaluated dihydroartemisinin/piperaquine. We conducted meta-analyses that included trials evaluating dihydroartemisinin/piperaquine plus cotrimoxazole, and trials that evaluated mefloquine plus cotrimoxazole, as we considered there to be no qualitative or quantitative heterogeneity among trials for most outcomes. We considered drug-related adverse events and HIV-related outcomes to be drug-specific. Daily cotrimoxazole prophylaxis plus another drug regimen (mefloquine or dihydroartemisinin/piperaquine) probably results in lower risk of maternal peripheral parasitaemia at delivery (RR 0.62, 95% CI 0.41 to 0.95; 2406 participants, 5 trials; moderate-certainty evidence). It results in little or no difference in maternal anaemia cases at delivery (RR 0.98, 95% CI 0.90 to 1.07; 2417 participants, 3 trials; high-certainty evidence). It probably results in a decrease in placental malaria measured by blood smear (RR 0.54, 95% CI 0.31 to 0.93; 1337 participants, 3 trials; moderate-certainty evidence), and probably results in little or no difference in low birth weight (RR 1.16, 95% CI 0.95 to 1.41; 2915 participants, 5 trials; moderate-certainty evidence). There is insufficient evidence to ascertain whether daily cotrimoxazole prophylaxis plus another drug regimen affects the risk of cord blood parasitaemia (RR 0.27, 95% CI 0.04 to 1.64; 2696 participants, 5 trials; very low-certainty evidence). Daily cotrimoxazole prophylaxis plus another drug regimen probably results in little or no difference in foetal loss (RR 1.03, 95% CI 0.73 to 1.46; 2957 participants, 5 trials; moderate-certainty evidence), and may result in little or no difference in neonatal mortality (RR 1.21, 95% CI 0.68 to 2.14; 2706 participants, 4 trials; low-certainty evidence). Due to the probability of an increased risk of mother-to-child HIV transmission and some adverse drug effects noted with mefloquine, we also looked at the results for dihydroartemisinin/piperaquine specifically. Dihydroartemisinin/piperaquine plus daily contrimoxazole probably results in little to no difference in maternal peripheral parasitaemia (RR 0.59, 95% CI 0.31 to 1.11; 1517 participants, 3 trials; moderate-certainty evidence) or anaemia at delivery (RR 0.95, 95% CI 0.82 to 1.10; 1454 participants, 2 trials; moderate-certainty evidence), but leads to fewer women having placental malaria when measured by histopathologic analysis (RR 0.67, 95% CI 0.50 to 0.90; 1570 participants, 3 trials; high-certainty evidence). The addition of dihydroartemisinin/piperaquine to daily cotrimoxazole probably made little to no difference to rates of low birth weight (RR 1.13, 95% CI 0.87 to 1.48; 1695 participants, 3 trials), foetal loss (RR 1.14, 95% CI 0.68 to 1.90; 1610 participants, 3 trials), or neonatal mortality (RR 1.03, 95% CI 0.39 to 2.72; 1467 participants, 2 trials) (all moderate-certainty evidence). We found low-certainty evidence of no increased risk of gastrointestinal drug-related adverse events (RR 1.42, 95% CI 0.51 to 3.98; 1447 participants, 2 trials) or mother-to-child HIV transmission (RR 1.54, 95% CI 0.26 to 9.19; 1063 participants, 2 trials).
Authors' conclusions: Dihydroartemisinin/piperaquine and mefloquine added to daily cotrimoxazole seem to be efficacious in preventing malaria infection in HIV-positive pregnant women compared to daily cotrimoxazole alone. However, increased risk of HIV transmission to the foetus and poor drug tolerability may be barriers to implementation of mefloquine in practice. In contrast, the evidence suggests that dihydroartemisinin/piperaquine does not increase the risk of HIV mother-to-child transmission and is well tolerated.
Trial registration: ClinicalTrials.gov NCT02524444 NCT02527005 NCT04158713 NCT00970879 NCT00126906 NCT00811421 NCT03671109 NCT00270530 NCT01746199 NCT00711906 NCT00209781 NCT02282293 NCT00131235 NCT00132535 NCT00164255 NCT03431168.
Copyright © 2024 The Authors. Cochrane Database of Systematic Reviews published by John Wiley & Sons, Ltd. on behalf of The Cochrane Collaboration.
Conflict of interest statement
CPD is author of a trial included in this review (González 2024), but was not involved in assessing the eligibility, risk of bias assessment, or analyses of this study. She has no known conflicts of interest.
CMC has no known conflicts of interest.
KEY has no known conflicts of interest.
VB is author of a trial that is included in this review (Manirakiza 2021), but was not involved in assessing the eligibility, risk of bias assessment, or analyses of this study. VB was Chair of the Data and Safety Monitoring Board of another trial included in this review (González 2024). She has no known conflicts of interest.
MJW has no known conflicts of interest.
RG is an author on two trials that are included in this review (González 2014; González 2024), but was not involved in assessing the eligibility, in risk of bias assessment, or analyses of these studies. She has no known conflicts of interest.
Figures
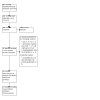
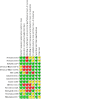

























































































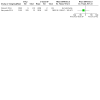


























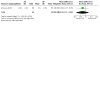











































Update of
-
Intermittent preventive treatment regimens for malaria in HIV-positive pregnant women.Cochrane Database Syst Rev. 2011 Oct 5;2011(10):CD006689. doi: 10.1002/14651858.CD006689.pub2. Cochrane Database Syst Rev. 2011. Update in: Cochrane Database Syst Rev. 2024 Sep 26;9:CD006689. doi: 10.1002/14651858.CD006689.pub3. PMID: 21975756 Free PMC article. Updated.
References
References to studies included in this review
Akinyotu 2018 {published data only}
-
- NCT02524444. A comparative study of mefloquine and S-P as prophylaxis against malaria in pregnant HIV + patients. clinicaltrials.gov/study/NCT02524444 (first posted on 12 August 2015).
Akinyotu 2019 {published and unpublished data}
-
- Akinyotu O, Bello F, Abdus-Salam R, Arowojolu A. A randomized controlled trial of azithromycin and sulphadoxine-pyrimethamine as prophylaxis against malaria in pregnancy among human immunodeficiency virus-positive women. Transactions of the Royal Society of Tropical Medicine and Hygiene 2019;113(8):463-70. [DOI: 10.1093/trstmh/trz028] - DOI - PubMed
-
- NCT02527005. A comparative study of azithromycin and S-P as prophylaxis in pregnant HIV+ patients. clinicaltrials.gov/study/NCT02527005 (first posted on 17 August 2015).
Barsosio 2024 {published and unpublished data}
-
- Barsosio HC, Madanitsa M, Ondieki ED, Dodd J, Onyango ED, Otieno K, et al. Chemoprevention for malaria with monthly intermittent preventive treatment with dihydroartemisinin-piperaquine in pregnant women living with HIV on daily co-trimoxazole in Kenya and Malawi: a randomised, double-blind, placebo-controlled trial. Lancet 2024;403(10424):365-78. [DOI: 10.1016/S0140-6736(23)02631-4] - DOI - PMC - PubMed
Denoeud‐Ndam 2014a {published and unpublished data}
-
- Denoeud-Ndam L, Zannou DM, Fourcade C, Taron-Brocard C, Porcher R, Atadokpede F, et al. Cotrimoxazole prophylaxis versus mefloquine intermittent preventive treatment to prevent malaria in HIV-infected pregnant women: two randomized controlled trials. Journal of Acquired Immune Deficiency Syndromes 2014;65(2):198-206. [DOI: 10.1097/QAI.0000000000000058] - DOI - PubMed
-
- NCT00970879. Prevention of pregnancy-associated malaria in HIV-infected women: cotrimoxazole prophylaxis versus mefloquine (PACOME). clinicaltrials.gov/study/NCT00970879 (first posted 2 September 2009).
Denoeud‐Ndam 2014b {published and unpublished data}
-
- Denoeud-Ndam L, Zannou DM, Fourcade C, Taron-Brocard C, Porcher R, Atadokpede F, et al. Cotrimoxazole prophylaxis versus mefloquine intermittent preventive treatment to prevent malaria in HIV-infected pregnant women: two randomized controlled trials. Journal of Acquired Immune Deficiency Syndromes 2014;65(2):198-206. [DOI: 10.1097/QAI.0000000000000058] - DOI - PubMed
-
- NCT00970879. Prevention of pregnancy-associated malaria in HIV-infected women: cotrimoxazole prophylaxis versus mefloquine (PACOME). clinicaltrials.gov/study/NCT00970879 (first posted 2 September 2009).
Filler 2006 {published data only}
-
- Filler SJ, Kazembe P, Thigpen M, Macheso A, Parise ME, Newman RD, et al. Randomized trial of 2-dose versus monthly sulfadoxine-pyrimethamine intermittent preventive treatment for malaria in HIV-positive and HIV-negative pregnant women in Malawi. Journal of Infectious Diseases 2006;194(3):286-93. [DOI: 10.1086/505080] - DOI - PubMed
-
- NCT00126906. Prevention of malaria during pregnancy using intermittent preventive treatment with sulfadoxine-pyrimethamine: Malawi. clinicaltrials.gov/study/NCT00126906 (first posted 3 August 2005).
González 2014 {published and unpublished data}
-
- González R, Desai M, Macete E, Ouma P, Kakolwa MA, Abdulla S, et al. Intermittent preventive treatment of malaria in pregnancy with mefloquine in HIV-infected women receiving cotrimoxazole prophylaxis: a multicenter randomized placebo-controlled trial. PLOS Medicine 2014;11(9):e1001735. [DOI: 10.1371/journal.pmed.1001735] - DOI - PMC - PubMed
-
- NCT00811421. Evaluation of alternative antimalarial drugs for malaria in pregnancy (MiPPAD). clinicaltrials.gov/study/NCT00811421 (first posted 18 December 2008).
González 2024 {published data only}
-
- González R, Nhampossa T, Mombo-Ngoma G, Mischlinger J, Esen M, Tchouatieu AM, et al. Evaluation of the safety and efficacy of dihydroartemisinin-piperaquine for intermittent preventive treatment of malaria in HIV-infected pregnant women: protocol of a multicentre, two-arm, randomised, placebo-controlled, superiority clinical trial (MAMAH project). BMJ Open 2021;11(11):e053197. [DOI: 10.1136/bmjopen-2021-053197] - DOI - PMC - PubMed
-
- González R, Nhampossa T, Mombo-Ngoma G, Mischlinger J, Esen M, Tchouatieu AM, et al. Safety and efficacy of dihydroartemisinin-piperaquine for intermittent preventive treatment of malaria in pregnant women with HIV from Gabon and Mozambique: a randomised, double-blind, placebo-controlled trial. Lancet Infectious Diseases 2024;24(5):476-87. [DOI: 10.1016/S1473-3099(23)00738-7] - DOI - PubMed
Hamer 2007 {published data only}
-
- Hamer DH, Mwanakasale V, Macleod WB, Chalwe V, Mukwamataba D, Champo D, et al. Two-dose versus monthly intermittent preventive treatment of malaria with sulfadoxine-pyrimethamine in HIV-seropositive pregnant Zambian women. Journal of Infectious Diseases 2007;196(11):1585-94. [DOI: 10.1086/522142] - DOI - PubMed
-
- NCT00270530. Intermittent preventive treatment of malaria in HIV-seropositive pregnant women in Zambia. clinicaltrials.gov/study/NCT00270530 (first posted 23 December 2005).
Klement 2013 {published data only}ISRCTN98835811
-
- ISRCTN98835811. Cotrimoxazol to prevent malaria in HIV-infected pregnant women in sub-Saharan Africa. www.isrctn.com/ISRCTN98835811 (first posted 27 August 2012).
-
- Klement E, Pitché P, Kendjo E, Singo A, D'Almeida S, Akouete F, et al. Effectiveness of co-trimoxazole to prevent Plasmodium falciparum malaria in HIV-positive pregnant women in sub-Saharan Africa: an open-label, randomized controlled trial. Clinical Infectious Diseases 2014;58(5):651-9. [DOI: 10.1093/cid/cit806] - DOI - PubMed
Manirakiza 2021 {published data only}
-
- Manirakiza A, Tondeur L, Ketta MYB, Sepou A, Serdouma E, Gondje S, et al. Cotrimoxazole versus sulfadoxine–pyrimethamine for intermittent preventive treatment of malaria in HIV-infected pregnant women in Bangui, Central African Republic: a pragmatic randomised controlled trial. Tropical Medicine & International Health 2021;26(10):1314-23. [DOI: 10.1111/tmi.13668] - DOI - PubMed
-
- NCT01746199. Efficacy of antifolates against malaria in HIV-infected pregnant women and the emergence of induced resistance in Plasmodium falciparum (MACOMBA). clinicaltrials.gov/study/NCT01746199 (first posted 6 December 2012).
Manyando 2014 {published data only}
-
- Manyando C, Njunju EM, Mwakazanga D, Chongwe G, Mkandawire R, Champo D, et al. Safety of daily co-trimoxazole in pregnancy in an area of changing malaria epidemiology: a phase 3b randomized controlled clinical trial. PLOS One 2014;9(5):e96017. [DOI: 10.1371/journal.pone.0096017] - DOI - PMC - PubMed
-
- NCT00711906. Daily co-trimoxazole prophylaxis to prevent malaria in pregnancy. clinicaltrials.gov/study/NCT00711906 (first posted 8 July 2008).
Menéndez 2008 {published data only}
-
- Menéndez C, Bardají A, Sigauque B, Romagosa C, Sanz S, Serra-Casas E, et al. A randomized placebo-controlled trial of intermittent preventive treatment in pregnant women in the context of insecticide treated nets delivered through the antenatal clinic. PLOS One 2008;3(4):e1934. [DOI: 10.1371/journal.pone.0001934] - DOI - PMC - PubMed
-
- NCT00209781. IPTp plus ITNs for malaria control in pregnant women. clinicaltrials.gov/study/NCT00209781 (first posted 13 September 2005).
Natureeba 2017 {published data only}
-
- NCT02282293. Reducing the burden of malaria in HIV-infected pregnant women and their HIV-exposed children (PROMOTE-BC2). clinicaltrials.gov/study/NCT02282293 (first posted 3 November 2014).
-
- Natureeba P, Kakuru A, Muhindo M, Ochieng T, Ategeka J, Koss CA, et al. Intermittent preventive treatment with dihydroartemisinin-piperaquine for the prevention of malaria among HIV-infected pregnant women. Journal of Infectious Diseases 2017;216(1):29-35. [DOI: 10.1093/infdis/jix110] - DOI - PMC - PubMed
References to studies excluded from this review
Gill 2007 {published data only}
-
- Gill CJ, Macleod WB, Mwanakasale V, Chalwe V, Mwananyanda L, Champo D, et al. Inferiority of single-dose sulfadoxine-pyrimethamine intermittent preventive therapy for malaria during pregnancy among HIV-positive Zambian women. Journal of Infectious Diseases 2007;196(11):1577-84. [DOI: 10.1086/522137] - DOI - PubMed
Luntamo 2010 {published data only (unpublished sought but not used)}
-
- Luntamo M, Kulmala T, Mbewe B, Cheung YB, Maleta K, Ashorn P. Effect of repeated treatment of pregnant women with sulfadoxine-pyrimethamine and azithromycin on preterm delivery in Malawi: a randomized controlled trial. American Journal of Tropical Medicine and Hygiene 2010;83(6):1212-20. [DOI: 10.4269/ajtmh.2010.10-0264] - DOI - PMC - PubMed
-
- Luntamo M, Rantala AM, Meshnick SR, Cheung YB, Kulmala T, Maleta K, et al. The effect of monthly sulfadoxine-pyrimethamine, alone or with azithromycin, on PCR-diagnosed malaria at delivery: a randomized controlled trial. PLOS One 2012;7(7):e41123. [DOI: 10.1371/journal.pone.0041123] - DOI - PMC - PubMed
-
- NCT00131235. Gestational sulfadoxine-pyrimethamine and azithromycin treatment to prevent preterm birth. clinicaltrials.gov/study/NCT00131235 (first posted 16 August 2005).
Parise 1998 {published data only}
-
- Parise ME, Ayisi JG, Nahlen BL, Schultz LJ, Roberts JM, Misore A, et al. Efficacy of sulfadoxine-pyrimethamine for prevention of placental malaria in an area of Kenya with a high prevalence of malaria and human immunodeficiency virus infection. American Journal of Tropical Medicine and Hygiene 1998;59(5):813-22. [DOI: 10.4269/ajtmh.1998.59.813] - DOI - PubMed
References to ongoing studies
NCT00132535 {published data only}
-
- NCT00132535. Influence of chloroquine on HIV viral load among pregnant women in Uganda. clinicaltrials.gov/study/NCT00132535?tab=history (first posted 19 August 2005).
NCT00164255 {published data only}
-
- NCT00164255. Efficacy of combination therapy for prevention of effects of malaria during pregnancy. clinicaltrials.gov/study/NCT00164255 (first posted 9 September 2005).
NCT03431168 (PREMISE) {published data only}
-
- NCT03431168. A novel regimen to prevent malaria and STI in pregnant women with HIV (PREMISE) [The PREMISE trial: a novel regimen to prevent malaria and sexually transmitted infections in pregnant women with HIV]. clinicaltrials.gov/study/NCT03431168 (first posted 13 February 2018).
PACTR201612001901313 {published data only}PACTR201612001901313
-
- PACTR201612001901313. Effectiveness of the combination of dihydroartemisinin and piperaquine for prevention of falciparum malaria during pregnancy in Tanzania. pactr.samrc.ac.za/TrialDisplay.aspx?TrialID=1901 (first posted 2 December 2016).
Additional references
CDC 2019
-
- Centers for Diseases Control and Prevention. Update: new recommendations for mefloquine use in pregnancy. Available from cdc.gov/malaria/new_info/2011/mefloquine_pregnancy.html (accessed 19 February 2023).
Denoeud‐Ndam 2013
Desai 2007
-
- Desai M, Ter Kuile FO, Nosten F, McGready R, Asamoa K, Brabin B, et al. Epidemiology and burden of malaria in pregnancy. Lancet Infectious Diseases 2007;7(2):93-104. [PMID: ] - PubMed
Desai 2015
-
- Desai M, Gutman J, L'lanziva A, Otieno K, Juma E, Kariuki S, et al. Intermittent screening and treatment or intermittent preventive treatment with dihydroartemisinin-piperaquine versus intermittent preventive treatment with sulfadoxine-pyrimethamine for the control of malaria during pregnancy in western Kenya: an open-label, three-group, randomised controlled superiority trial. Lancet 2015;386(10012):2507-19. - PMC - PubMed
Eisele 2012
-
- Eisele TP, Larsen DA, Anglewicz PA, Keating J, Yukich J, Bennett A, et al. Malaria prevention in pregnancy, birthweight, and neonatal mortality: a meta-analysis of 32 national cross-sectional datasets in Africa. Lancet Infectious Diseases 2012;12(12):942-9. - PubMed
Figueroa‐Romero 2024
Gamble 2007
González 2014
-
- González R, Desai M, Macete E, Ouma P, Kakolwa MA, Abdulla S, et al. Intermittent preventive treatment of malaria in pregnancy with mefloquine in HIV-infected women receiving cotrimoxazole prophylaxis: a multicenter randomized placebo-controlled trial. PLOS Medicine 2014;11(9):e1001735. - PMC - PubMed
González 2016
González 2018
González 2012
-
- González R, Ataide R, Naniche D, Menéndez C, Mayor A. HIV and malaria interactions: where do we stand? Expert Review of Anti-Infective Therapy 2012;10(2):153-65. - PubMed
Gutman 2017
Higgins 2011
-
- Higgins JPT, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Kakuru 2016
Kamya 2012
Kwenti 2018
Manyando 2013
Mayor 2015
McKenzie 2023
-
- McKenzie JE, Brennan SE, Ryan RE, Thomson HJ, Johnston RV. Chapter 9: Summarizing study characteristics and preparing for synthesis. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.4 (updated August 2023). Cochrane, 2023.
Menéndez 2010
Menéndez 2011
-
- Menéndez C, Serra-Casas E, Scahill MD, Sanz S, Nhabomba A, Bardají A, et al. HIV and placental infection modulate the appearance of drug-resistant Plasmodium falciparum in pregnant women who receive intermittent preventive treatment. Clinical Infectious Diseases 2011;52(1):41-8. - PubMed
Moore 2017
-
- Moore KA, Simpson JA, Scoullar MJL, McGready R, Fowkes FJI. Quantification of the association between malaria in pregnancy and stillbirth: a systematic review and meta-analysis. Lancet Global Health 2017;5(11):e1101-12. - PubMed
Naing 2016
Ndam 2017
RevMan Web 2023 [Computer program]
-
- Review Manager Web (RevMan Web). Version 6.4.2. The Cochrane Collaboration, 2023. Available at revman.cochrane.org.
Saito 2020
Sicuri 2010
Tran 2020
UNAIDS 2016
-
- UNAIDS. Global UNAIDS update 2016. unaids.org/sites/default/files/media_asset/global-AIDS-update-2016_en.pdf (accessed 14 August 2020).
UNAIDS 2019
-
- UNAIDS. Global AIDS update 2019: communities at the center. unaids.org/sites/default/files/media_asset/2019-global-AIDS-update_en.pdf (accessed 14 August 2020).
Van Eijk 2003
-
- Van Eijk AM, Ayisi JG, Ter Kuile FO, Misore AO, Otieno JA, Rosen DH, et al. HIV increases the risk of malaria in women of all gravidities in Kisumu, Kenya. AIDS (London, England) 2003;17(4):595-603. - PubMed
Vidya Vijayan 2017
Ward 2007
-
- Ward SA, Sevene EJ, Hastings IM, Nosten F, McGready R. Antimalarial drugs and pregnancy: safety, pharmacokinetics, and pharmacovigilance. Lancet Infectious Diseases 2007;7(2):136-44. - PubMed
White 2005
WHO 2012
-
- World Health Organization. Updated WHO policy recommendation: intermittent preventive treatment of malaria in pregnancy using sulfadoxine-pyrimethamine (IPTp-SP). 2012. Available from who.int/malaria/publications/atoz/who_iptp_sp_policy_recommendation/en/ (accessed 19 February 2023).
WHO 2015
-
- World Health Organization. Guidelines for the treatment of malaria. Third edition. 2015. Available from who.int/malaria/publications/atoz/9789241549127/en/ (accessed 19 February 2023).
WHO 2016
-
- World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection, 2nd edition. 2016. Available from who.int/hiv/pub/arv/arv-2016/en/ (accessed 19 February 2023).
WHO 2017
-
- World Health Organization. Malaria in HIV/AIDS patients. 2017. Available from who.int/malaria/areas/high_risk_groups/hiv_aids_patients/en/ (accessed 19 February 2023).
WHO 2022a
-
- World Health Organization. World malaria report 2022. Available from who.int/teams/global-malaria-programme/reports/world-malaria-report-2022 (accessed 19 February 2023).
WHO 2022b
-
- World Health Organization. Guidelines for malaria. 2022. Available from who.int/publications/i/item/guidelines-for-malaria (accessed 19 February 2023).
WHO 2023
-
- World Health Organization. World Malaria Report 2023. Available from www.who.int/teams/global-malaria-programme/reports/world-malaria-report-... (accessed June 2024).
References to other published versions of this review
CRD42021233901
-
- CRD42021233901. Intermittent preventive treatment regimens for malaria in HIV-positive pregnant women. www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021233901 (first received 28 January 2021).
Mathanga 2007
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

