Innate cells and STAT1-dependent signals orchestrate vaccine-induced protection against invasive Cryptococcus infection
- PMID: 39324785
- PMCID: PMC11481872
- DOI: 10.1128/mbio.01944-24
Innate cells and STAT1-dependent signals orchestrate vaccine-induced protection against invasive Cryptococcus infection
Abstract
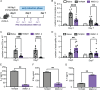
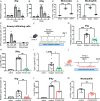
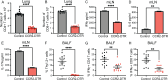
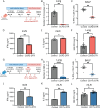
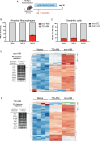
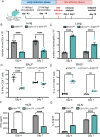
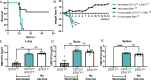
Fungal pathogens are underappreciated causes of significant morbidity and mortality worldwide. In previous studies, we determined that a heat-killed, Cryptococcus neoformans fbp1-deficient strain (HK-fbp1) is a potent vaccine candidate. We determined that vaccination with HK-fbp1 confers protective immunity against lethal Cryptococcosis in an interferon γ (IFNγ)-dependent manner. In this study, we set out to uncover cellular sources and relevant targets of the protective effects of IFNγ in response to the HK-fbp1 vaccine. We found that early IFNγ production peaks at day 3 and that monocytes and neutrophils are important sources of this cytokine after vaccination. Neutralization of IFNγ at day 3 results in impaired CCR2+ monocyte recruitment and reduced differentiation into monocyte-derived dendritic cells (Mo-DC). In turn, depletion of CCR2+ cells prior to immunization results in impaired activation of IFNγ-producing CD4 and CD8 T cells. Thus, monocytes are important targets of innate IFNγ and help promote further IFNγ production by lymphocytes. We employed monocyte-fate mapper and conditional STAT1 knockout mice to uncover that STAT1 activation in CD11c+ cells, including alveolar macrophages, Mo-DCs, and monocyte-derived macrophages (Mo-Mac) is essential for HK-fbp1 vaccine-induced protection. Altogether, our aggregate findings suggest critical roles for innate cells as orchestrators of vaccine-induced protection against Cryptococcus infection.IMPORTANCEThe number of patients susceptible to invasive fungal infections across the world continues to rise at an alarming pace yet current antifungal drugs are often inadequate. Immune-based interventions and novel antifungal vaccines hold the promise of significantly improving patient outcomes. In previous studies, we identified a Cryptococcus neoformans mutant strain (Fbp1-deficient) as a potent, heat-inactivated vaccine candidate capable of inducing homologous and heterologous antifungal protection. In this study, we used a combination of methods together with a cohort of conditional knockout mouse strains to interrogate the roles of innate cells in the orchestration of vaccine-induced antifungal protection. We uncovered novel roles for neutrophils and monocytes as coordinators of a STAT1-dependent cascade of responses that mediate vaccine-induced protection against invasive cryptococcosis. This new knowledge will help guide the future development of much-needed antifungal vaccines.
Keywords: Cryptococcus neoformans; antifungal therapy; immunization; innate immunity; monocytes; neutrophils.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
A Heat-Killed Cryptococcus Mutant Strain Induces Host Protection against Multiple Invasive Mycoses in a Murine Vaccine Model.mBio. 2019 Nov 26;10(6):e02145-19. doi: 10.1128/mBio.02145-19. mBio. 2019. PMID: 31772051 Free PMC article.
-
Immunological correlates of protection mediated by a whole organism, Cryptococcus neoformans, vaccine deficient in chitosan.mBio. 2024 Aug 14;15(8):e0174624. doi: 10.1128/mbio.01746-24. Epub 2024 Jul 9. mBio. 2024. PMID: 38980038 Free PMC article.
-
Development of a Heat-Killed fbp1 Mutant Strain as a Therapeutic Agent To Treat Invasive Cryptococcus Infection.Microbiol Spectr. 2023 Jan 31;11(2):e0495522. doi: 10.1128/spectrum.04955-22. Online ahead of print. Microbiol Spectr. 2023. PMID: 36719231 Free PMC article.
-
Innate host defenses against Cryptococcus neoformans.J Microbiol. 2016 Mar;54(3):202-11. doi: 10.1007/s12275-016-5625-7. Epub 2016 Feb 27. J Microbiol. 2016. PMID: 26920880 Review.
-
Feasibility and prospects for a vaccine to prevent cryptococcosis.Med Mycol. 2005 Dec;43(8):667-80. doi: 10.1080/13693780500448230. Med Mycol. 2005. PMID: 16422296 Review.
Cited by
-
Research progress on immunometabolism and gut microbiota in cryptococcal meningitis: mechanisms and therapeutic implications.Front Neurosci. 2025 Jul 16;19:1622349. doi: 10.3389/fnins.2025.1622349. eCollection 2025. Front Neurosci. 2025. PMID: 40740256 Free PMC article. Review.
-
Will the Real Immunogens Please Stand Up: Exploiting the Immunogenic Potential of Cryptococcal Cell Antigens in Fungal Vaccine Development.J Fungi (Basel). 2024 Dec 4;10(12):840. doi: 10.3390/jof10120840. J Fungi (Basel). 2024. PMID: 39728336 Free PMC article. Review.
-
Spatial organization of pulmonary type 2 inflammation by a macrophage-derived cholesterol metabolite.bioRxiv [Preprint]. 2025 Aug 1:2025.07.29.666625. doi: 10.1101/2025.07.29.666625. bioRxiv. 2025. PMID: 40766699 Free PMC article. Preprint.
-
Improved Broad Spectrum Antifungal Drug Synergies with Cryptomycin, a Cdc50-Inspired Antifungal Peptide.ACS Infect Dis. 2024 Nov 8;10(11):3973-3993. doi: 10.1021/acsinfecdis.4c00681. Epub 2024 Oct 29. ACS Infect Dis. 2024. PMID: 39475550 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

