Synergistic blockade of TIGIT and PD-L1 increases type-1 inflammation and improves parasite control during murine blood-stage Plasmodium yoelii non-lethal infection
- PMID: 39324794
- PMCID: PMC11556036
- DOI: 10.1128/iai.00345-24
Synergistic blockade of TIGIT and PD-L1 increases type-1 inflammation and improves parasite control during murine blood-stage Plasmodium yoelii non-lethal infection
Abstract
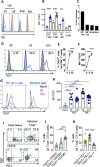
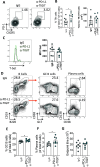
Pro-inflammatory immune responses are rapidly suppressed during blood-stage malaria but the molecular mechanisms driving this regulation are still incompletely understood. In this study, we show that the co-inhibitory receptors TIGIT and PD-1 are upregulated and co-expressed by antigen-specific CD4+ T cells (ovalbumin-specific OT-II cells) during non-lethal Plasmodium yoelii expressing ovalbumin (PyNL-OVA) blood-stage infection. Synergistic blockade of TIGIT and PD-L1, but not individual blockade of each receptor, during the early stages of infection significantly improved parasite control during the peak stages (days 10-15) of infection. Mechanistically, this protection was correlated with significantly increased plasma levels of IFN-γ, TNF, and IL-2, and an increase in the frequencies of IFN-γ-producing antigen-specific T-bet+ CD4+ T cells (OT-II cells), but not antigen-specific CD8+ T cells (OT-I cells), along with expansion of the splenic red pulp and monocyte-derived macrophage populations. Collectively, our study identifies a novel role for TIGIT in combination with the PD1-PD-L1 axis in regulating specific components of the pro-inflammatory immune response and restricting parasite control during the acute stages of blood-stage PyNL infection.
Keywords: CD4+ T cell; Plasmodium; checkpoint molecules; immune regulation; malaria.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Combinatorial Tim-3 and PD-1 activity sustains antigen-specific Th1 cell numbers during blood-stage malaria.Parasite Immunol. 2020 Sep;42(9):e12723. doi: 10.1111/pim.12723. Epub 2020 May 25. Parasite Immunol. 2020. PMID: 32306409
-
Blockade of LAG-3 in PD-L1-Deficient Mice Enhances Clearance of Blood Stage Malaria Independent of Humoral Responses.Front Immunol. 2021 Jan 14;11:576743. doi: 10.3389/fimmu.2020.576743. eCollection 2020. Front Immunol. 2021. PMID: 33519801 Free PMC article.
-
Fluctuations of Spleen Cytokine and Blood Lactate, Importance of Cellular Immunity in Host Defense Against Blood Stage Malaria Plasmodium yoelii.Front Immunol. 2019 Sep 25;10:2207. doi: 10.3389/fimmu.2019.02207. eCollection 2019. Front Immunol. 2019. PMID: 31608052 Free PMC article.
-
Macrophage-mediated but gamma interferon-independent innate immune responses control the primary wave of Plasmodium yoelii parasitemia.Infect Immun. 2007 Dec;75(12):5806-18. doi: 10.1128/IAI.01005-07. Epub 2007 Oct 8. Infect Immun. 2007. PMID: 17923512 Free PMC article.
-
MAPK phosphotase 5 deficiency contributes to protection against blood-stage Plasmodium yoelii 17XL infection in mice.J Immunol. 2014 Apr 15;192(8):3686-96. doi: 10.4049/jimmunol.1301863. Epub 2014 Mar 14. J Immunol. 2014. PMID: 24634491
References
-
- Kumar R, Loughland JR, Ng SS, Boyle MJ, Engwerda CR. 2020. The regulation of CD4(+) T cells during malaria. Immunol Rev 293:70–87. doi: 10.1111/imr.12804 - DOI - PubMed
-
- Abel A, Steeg C, Aminkiah F, Addai-Mensah O, Addo M, Gagliani N, Casar C, Yar DD, Owusu-Dabo E, Jacobs T, Mackroth MS. 2018. Differential expression pattern of co-inhibitory molecules on CD4+ T cells in uncomplicated versus complicated malaria. Sci Rep 8:4789. doi:10.1038/s41598-018-22659-1 - DOI - PMC - PubMed
-
- Brandi J, Lehmann C, Kaminski LC, Schulze Zur Wiesch J, Addo M, Ramharter M, Mackroth M, Jacobs T, Riehn M. 2022. T cells expressing multiple co-inhibitory molecules in acute malaria are not exhausted but exert a suppressive function in mice. Eur J Immunol 52:312–327. doi:10.1002/eji.202149424 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

