Naamidine A reveals a promising zinc-binding strategy for topical antifungal therapy
- PMID: 39324798
- PMCID: PMC11539223
- DOI: 10.1128/aac.01194-24
Naamidine A reveals a promising zinc-binding strategy for topical antifungal therapy
Abstract
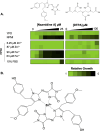
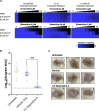
Fungal disease affects over a billion people worldwide. Naamidine A inhibits the growth of diverse fungal pathogens through an unknown mechanism. Here, we show that the supplementation of medium with excess zinc abolishes the antifungal activity of naamidine A. Furthermore, we highlight that naamidine A has in vitro activity against terbinafine-resistant Trichophyton spp. and in vivo efficacy in a mouse model of dermatomycosis caused by T. mentagrophytes, highlighting its therapeutic potential as a topical treatment.
Keywords: Candida albicans; Trichophyton; dermatomycosis; drug-resistant; metal chelation; natural product; zinc.
Conflict of interest statement
L.E.C. and L.W. are co-founders and shareholders in Bright Angel Therapeutics, a platform company for the development of novel antifungal therapeutics. L.E.C. is a Science Advisor for Kapoose Creek, a company that harnesses the therapeutic potential of fungi.
Figures
References
-
- Fisher MC, Alastruey-Izquierdo A, Berman J, Bicanic T, Bignell EM, Bowyer P, Bromley M, Brüggemann R, Garber G, Cornely OA, Gurr SJ, Harrison TS, Kuijper E, Rhodes J, Sheppard DC, Warris A, White PL, Xu J, Zwaan B, Verweij PE. 2022. Tackling the emerging threat of antifungal resistance to human health. Nat Rev Microbiol 20:557–571. doi:10.1038/s41579-022-00720-1 - DOI - PMC - PubMed
-
- Martínez-Fructuoso L, Arends SJR, Freire VF, Evans JR, DeVries S, Peyser BD, Akee RK, Thornburg CC, Kumar R, Ensel S, Morgan GM, McConachie GD, Veeder N, Duncan LR, Grkovic T, O’Keefe BR. 2023. Screen for new antimicrobial natural products from the NCI program for natural product discovery prefractionated extract library. ACS Infect Dis 9:1245–1256. doi:10.1021/acsinfecdis.3c00067 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

