Orientia tsutsugamushi infection reduces host gluconeogenic but not glycolytic substrates
- PMID: 39324805
- PMCID: PMC11556148
- DOI: 10.1128/iai.00284-24
Orientia tsutsugamushi infection reduces host gluconeogenic but not glycolytic substrates
Abstract
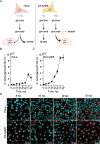
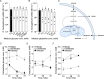
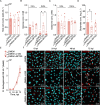
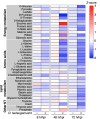
Orientia tsutsugamushi a causal agent of scrub typhus, is an obligate intracellular bacterium that, akin to other rickettsiae, is dependent on host cell-derived nutrients for survival and thus pathogenesis. Based on limited experimental evidence and genome-based in silico predictions, O. tsutsugamushi is hypothesized to parasitize host central carbon metabolism (CCM). Here, we (re-)evaluated O. tsutsugamushi dependency on host cell CCM as initiated by glucose and glutamine. Orientia infection had no effect on host glucose and glutamine consumption or lactate accumulation, indicating no change in overall flux through CCM. However, host cell mitochondrial activity and ATP levels were reduced during infection and correspond with lower intracellular glutamine and glutamate pools. To further probe the essentiality of host CCM in O. tsutsugamushi proliferation, we developed a minimal medium for host cell cultivation and paired it with chemical inhibitors to restrict the intermediates and processes related to glucose and glutamine metabolism. These conditions failed to negatively impact O. tsutsugamushi intracellular growth, suggesting the bacterium is adept at scavenging from host CCM. Accordingly, untargeted metabolomics was utilized to evaluate minor changes in host CCM metabolic intermediates across O. tsutsugamushi infection and revealed that pathogen proliferation corresponds with reductions in critical CCM building blocks, including amino acids and TCA cycle intermediates, as well as increases in lipid catabolism. This study directly correlates O. tsutsugamushi proliferation to alterations in host CCM and identifies metabolic intermediates that are likely critical for pathogen fitness.IMPORTANCEObligate intracellular bacterial pathogens have evolved strategies to reside and proliferate within the eukaryotic intracellular environment. At the crux of this parasitism is the balance between host and pathogen metabolic requirements. The physiological basis driving O. tsutsugamushi dependency on its mammalian host remains undefined. By evaluating alterations in host metabolism during O. tsutsugamushi proliferation, we discovered that bacterial growth is independent of the host's nutritional environment but appears dependent on host gluconeogenic substrates, including amino acids. Given that O. tsutsugamushi replication is essential for its virulence, this study provides experimental evidence for the first time in the post-genomic era of metabolic intermediates potentially parasitized by a scrub typhus agent.
Keywords: Orientia tsutsugamushi; Rickettsiales; amino acid parasitism; central carbon metabolism; intracellular pathogen; nutrient parasitism; obligate intracellular bacterium; rickettsial disease; scrub typhus; untargeted metabolomics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Orientia tsutsugamushi Strain Ikeda Ankyrin Repeat-Containing Proteins Recruit SCF1 Ubiquitin Ligase Machinery via Poxvirus-Like F-Box Motifs.J Bacteriol. 2015 Oct;197(19):3097-109. doi: 10.1128/JB.00276-15. Epub 2015 Jul 13. J Bacteriol. 2015. PMID: 26170417 Free PMC article.
-
Orientia tsutsugamushi ankyrin repeat-containing protein family members are Type 1 secretion system substrates that traffic to the host cell endoplasmic reticulum.Front Cell Infect Microbiol. 2015 Feb 3;4:186. doi: 10.3389/fcimb.2014.00186. eCollection 2014. Front Cell Infect Microbiol. 2015. PMID: 25692099 Free PMC article.
-
An Alternative Splicing Variant of the Mixed-Lineage Leukemia 5 Protein Is a Cellular Adhesion Receptor for ScaA of Orientia tsutsugamushi.mBio. 2023 Feb 28;14(1):e0154322. doi: 10.1128/mbio.01543-22. Epub 2022 Dec 21. mBio. 2023. PMID: 36541760 Free PMC article.
-
An Update on Host-Pathogen Interplay and Modulation of Immune Responses during Orientia tsutsugamushi Infection.Clin Microbiol Rev. 2018 Jan 31;31(2):e00076-17. doi: 10.1128/CMR.00076-17. Print 2018 Apr. Clin Microbiol Rev. 2018. PMID: 29386235 Free PMC article. Review.
-
Orientia tsutsugamushi: An Unusual Intracellular Bacteria-Adaptation Strategies, Available Antibiotics, and Alternatives for Treatment.Curr Microbiol. 2024 Jun 21;81(8):236. doi: 10.1007/s00284-024-03754-1. Curr Microbiol. 2024. PMID: 38907107 Review.
Cited by
-
Orientia tsutsugamushi Modulates RIPK3 Cellular Levels but Does Not Inhibit Necroptosis.Pathogens. 2025 May 14;14(5):478. doi: 10.3390/pathogens14050478. Pathogens. 2025. PMID: 40430799 Free PMC article.
-
Orientia tsutsugamushi alters the intranuclear balance of cullin-1 and c-MYC to inhibit apoptosis.Infect Immun. 2025 Mar 11;93(3):e0055924. doi: 10.1128/iai.00559-24. Epub 2025 Feb 20. Infect Immun. 2025. PMID: 39976440 Free PMC article.
-
Orientia tsutsugamushi modulates p53, the cell cycle, and genotoxicity to maintain its intracellular niche.Nat Commun. 2025 Aug 19;16(1):7728. doi: 10.1038/s41467-025-63149-z. Nat Commun. 2025. PMID: 40830139 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

